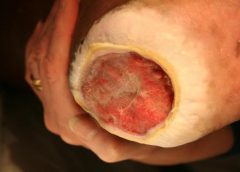
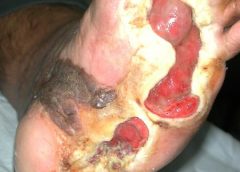
Safety and Efficacy Study of VM202 in the Treatment of Chronic Non-Healing Foot Ulcers. This study will assess the safety and efficacy of using gene therapy via intramuscular injections of the calf for patients with chronic non-healing foot ulcers.
The first patient has been dosed in a Phase III trial assessing ViroMed’s VM202, the first pivotal study of a gene therapy indicated for patients with nonhealing diabetic foot ulcers (NHU) and concomitant peripheral artery disease (PAD).
The Phase III trial (NCT02563522) is a double-blind, placebo-controlled, multicenter study designed to evaluate VM202 for safety and efficacy in 300 adults with a diabetic foot ulcer and concomitant PAD. Two hundred patients will be randomized to VM202 and the other 100 to placebo, ViroMed’s U.S. division VM BioPharma said yesterday. (more…)
Read More


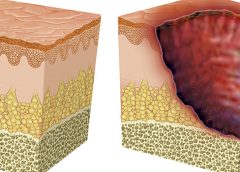
 Washington, D.C. – The National Pressure Ulcer Advisory Panel (NPUAP) announced the convening of its subcommittee, the Support Surface Standards Initiative (S3I), this spring to approve new standards tests that may help prevent pressure injuries in bed-bound individuals.
Washington, D.C. – The National Pressure Ulcer Advisory Panel (NPUAP) announced the convening of its subcommittee, the Support Surface Standards Initiative (S3I), this spring to approve new standards tests that may help prevent pressure injuries in bed-bound individuals.