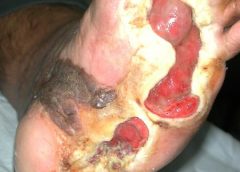
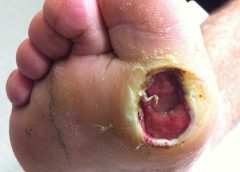
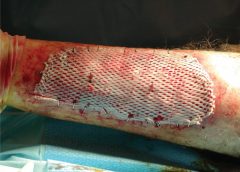
Safety and Efficacy Study of VM202 in the Treatment of Chronic Non-Healing Foot Ulcers. This study will assess the safety and efficacy of using gene therapy via intramuscular injections of the calf for patients with chronic non-healing foot ulcers.
The first patient has been dosed in a Phase III trial assessing ViroMed’s VM202, the first pivotal study of a gene therapy indicated for patients with nonhealing diabetic foot ulcers (NHU) and concomitant peripheral artery disease (PAD).
The Phase III trial (NCT02563522) is a double-blind, placebo-controlled, multicenter study designed to evaluate VM202 for safety and efficacy in 300 adults with a diabetic foot ulcer and concomitant PAD. Two hundred patients will be randomized to VM202 and the other 100 to placebo, ViroMed’s U.S. division VM BioPharma said yesterday. (more…)
Read More