BY: NANCY MORGAN, RN, BSN, MBA, WOCN, WCC, CWCMS, DWC
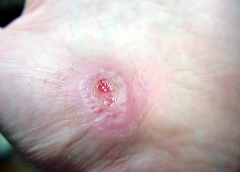
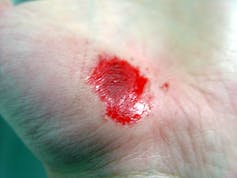
You’ve identified your patient’s lower extremity ulcer as a venous ulcer. It has irregular edges, a ruddy wound base, and a moderate amount of drainage. The patient’s bilateral lower extremities are edematous. As a wound care clinician, you know sustained graduated compression is key to healing stasis ulcers and preventing their recurrence. (more…)
Search Results for: abi
A Saudi rehabilitation facility fights pressure ulcers
By Joanne Aspiras Jovero, BSEd, BSN, RN; Hussam Al-Nusair, MSc Critical Care, ANP, RN; and Marilou Manarang, BSN, RN
A common problem in long-term care facilities, pressure ulcers are linked to prolonged hospitalization, pain, social isolation, sepsis, and death. This article explains how a Middle East rehabilitation facility battles pressure ulcers with the latest evidence-based practices, continual staff education, and policy and procedure updates. Sultan Bin Abdulaziz Humanitarian City (SBAHC) in Riyadh, Saudi Arabia, uses an interdisciplinary approach to address pressure-ulcer prevention and management. This article describes the programs, strategies, and preventive measures that have reduced pressure-ulcer incidence. (more…)
Read MoreWhen you can’t rely on ABIs
By Robyn Bjork, MPT, CWS, WCC, CLT-LANA
One of the worst fears of a wound care clinician is inadvertently compressing a leg with critical limb ischemia—a condition marked by barely enough blood flow to sustain tissue life. Compression (as well as infection or injury) could lead to necrosis, the need for amputation, or even death. The gold standard of practice is to obtain an ankle-brachial index (ABI) before applying compression. However, recent research and expert opinion indicate an elevated or normal ABI is deceptive in patients with advanced diabetes. What’s worse, in the diabetic foot, skin may die from chronic capillary ischemia even when total blood perfusion is normal. For information on how to perform an ABI and interpret results, click on this link. (more…)
Read MoreWhy do older people heal more slowly?
By Matthew Steinhauser, University of Pittsburgh
I recently visited an 83-year-old patient in the hospital after EMTs rushed her to the ER with an infected leg wound. Her ordeal started inconspicuously when she bumped into the sharp edge of a table and developed a small cut. The patient’s wound didn’t close, but she ignored it until she woke up in pain one morning two weeks after first injuring her leg. Her daughter called 911 after noticing angry, red skin discoloration and pus – both signs of an infection. Our medical team treated her with IV antibiotics and cleared up the infection, but the wound did not fully close until at least a month later, well after she was discharged from the hospital.
How different the story is when children get a cut. They may scream initially, but within days, the scab falls off, revealing new skin. Why was healing so delayed in my 83-year-old patient compared to a healthy child?
The answer is age. Decades of life slow down healing for most tissues, and wounds in skin can offer a window into why this slowdown occurs.
Three stages of wound healing
I am physician who studies how aging predisposes patients to diseases like diabetes and whether behavioral changes such as intermittent fasting may slow down aging. In order to understand why the skin wound in my older patient healed so slowly, it is important to first understand how wounds heal under the ideal conditions of youth.
The wound healing process is classically categorized into three stages.
Right after a wound occurs, the inflammatory response begins.
Jpbarrass via Wikimedia Commons
The first stage is inflammation, essentially the body’s attempt to clean the wound. During the inflammatory phase, immune cells called phagocytes move into the wound, kill any contaminating bacteria, and ingest and dispose of dead cells and debris.
Jpbarrass via Wikimedia Commons
Inflammation sets the stage for the regenerative phase, where several processes work in concert to regrow damaged skin. Replacement skin cells are born when cells at the edge of the wound divide, while fibroblast cells lay down a supportive scaffolding called the extracellular matrix. This holds the new cells together. Any damaged supporting structures of the skin, such as the blood vessels that supply critical oxygen and nutrients, also need to regrow. The second stage effectively closes the wound and restores a protective barrier against bacteria.
Jpbarrass via Wikimedia Commons
The regenerative phase is a relatively quick, but tenuous fix – new skin is fragile. The final remodeling phase plays out over a couple of years as the new skin is progressively strengthened by several parallel processes. The extracellular matrix, which was initially laid down in a haphazard fashion, is broken down and replaced in a more durable way. Any residual cells from prior phases that are no longer needed – such as immune cells or fibroblasts – become inactive or die. In addition to strengthening the new skin, these collective actions also account for the tendency of scars to visibly fade with time.
Products
Diseases disrupt the healing process
One major way aging can derail the orderly and efficient progression through the stages of healing is through the health problems that stem from diseases of old age.
Diabetes is one example of a disease that is strongly associated with older age. One of the many ways that diabetes negatively affects healing is by causing blood vessels to narrow. As a consequence of inadequate circulation, crucial nutrients and oxygen do not reach the wound in sufficient quantities to fuel the second regenerative phase.
Diabetes is just one of many age-related diseases that disrupts normal processes in the body such as wound healing.
Cells age too
Aside from the negative impacts of age-associated diseases, cells themselves age. In an extreme sign of aging called cellular senescence, cells permanently lose the ability to divide. Senescent cells accumulate in skin and many other organs as people age and cause a host of problems.
When cells divide more slowly – or when they stop dividing altogether due to senescence – skin becomes thinner. The replacement of fat cells, which form a cushioning layer under the skin, also declines with age. The skin of older patients is therefore more prone to injury in the first place.
Once an older person’s skin is injured, the skin has a harder time healing properly as well. Aging and senescent immune cells cannot defend against bacteria, and the risk of serious skin infection rises. Then in the regenerative stage, slow rates of cell division translate into slow skin regrowth. My patient exhibited all of these negative effects of age – her thin, almost translucent skin ruptured from a minor bump, became infected and took nearly two months to fully regrow.
But senescent cells are more than just dysfunctional bystanders. For reasons that are not yet fully understood, senescent cells release toxic byproducts that damage surrounding tissue and drive inflammation – even when there’s no bacterial threat present. Some of these byproducts can even accelerate senescence in neighboring cells. This suggests that intrinsic aging of cells is in essence contagious and senescent cells actively fuel an uncontrolled cycle of inflammation and tissue damage that further impedes successful regeneration and healing.
A whole body problem
As the most outwardly visible tissue of the body, the skin provides a window into why people heal more slowly with age, but all tissues can be injured and are susceptible to the effects of aging. Injuries may be small, repetitive and build up over time – like the effect of smoking on the lungs. Or they may be discrete and dramatic – such as the death of heart cells with a heart attack. Different tissues may heal in different ways. Yet all tissues share a sensitivity to the repercussions of an aging immune system and a decline in the ability to regrow dead or damaged cells.
Understanding why healing slows down with age is important, but my patient asked a very practical question that physicians often face in one form or another: “Doctor, what can you do for me?”
Unfortunately, current treatment of wounds is fairly old-fashioned and often ineffective. Some of the options available include wound dressing changes, antibiotics when the wound is infected or treatment in a high oxygen chamber when circulation is bad due to diabetes.
There is hope, though, that medicine can do better and that progress in understanding the aging process will lead to new therapies. Neutralizing senescent cells in mice, for example, improves a variety of age-associated diseases. While it is way too early to say that researchers have discovered the fountain of youth, I am optimistic for a future when physicians will bend the aging curve and make skin and other organs heal faster and better.
Matthew Steinhauser, Associate Professor of Medicine, University of Pittsburgh
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Leprosy cases are rising in the US – what is the ancient disease and why is it spreading now?
By Robert A. Schwartz, Rutgers University
The word “leprosy” conjures images of biblical plagues, but the disease is still with us today. Caused by infectious bacteria, some 200,000 new cases are reported each year, according to the World Health Organization. In the United States, leprosy has been entrenched for more than a century in parts of the South where people came into contact with armadillos, the principle proven linkage from animal to humans. However, the more recent outbreaks in the Southeast, especially Florida, have not been associated with animal exposure.
The Conversation talked with Robert A. Schwartz, professor and head of dermatology at Rutgers New Jersey Medical School, to explain what researchers know about the disease.
What is leprosy and why is it resurfacing in the US?
Leprosy is caused by two different but similar bacteria — Mycobacterium leprae and Mycobacterium lepromatosis — the latter having just been identified in 2008. Leprosy, also known as Hansen’s disease, is avoidable. Transmission among the most vulnerable in society, including migrant and impoverished populations, remains a pressing issue.
This age-old neglected tropical disease, which is still present in more than 120 countries, is now a growing challenge in parts of North America.
Leprosy is beginning to occur regularly within parts of the southeastern United States. Most recently, Florida has seen a heightened incidence of leprosy, accounting for many of the newly diagnosed cases in the U.S.
The surge in new cases in central Florida highlights the urgent need for health care providers to report them immediately. Contact tracing is critical to identifying sources and reducing transmission.
Traditional risk factors include zoonotic exposure and having recently lived in leprosy-endemic countries. Brazil, India and Indonesia have each noted more than 10,000 new cases since 2019, according to the World Health Organization data, and more than a dozen countries have reported between 1,000 to 10,000 new cases over the same time period.
Why was leprosy stigmatized in biblical times?
Evidence suggests that leprosy has plagued civilization since at least the second millennium B.C.
From that time until the mid-20th century, limited treatments were available, so the bacteria could infiltrate the body and cause prominent physical deformities such as disfigured hands and feet. Advanced cases of leprosy cause facial features resembling that of a lion in humans.
Many mutilating and distressing skin disorders such as skin cancers and deep fungal infections were also confused with leprosy by the general public.
Fear of contagion has led to tremendous stigmatization and social exclusion. It was such a serious concern that the Kingdom of Jerusalem had a specialized hospital to care for those suffering from leprosy.
How infectious is leprosy?
Research shows that prolonged in-person contact via respiratory droplets is the primary mode of transmission, rather than through normal, everyday contact such as embracing, shaking hands or sitting near a person with leprosy. People with leprosy generally do not transmit the disease once they begin treatment.
Armadillos represent the only known zoonotic reservoir of leprosy-causing bacteria that threaten humans. These small mammals are common in Central and South America and in parts of Texas, Louisiana, Missouri and other states, where they are sometimes kept as pets or farmed as meat. Eating armadillo meat is not a clear cause of leprosy, but capturing and raising armadillos, along with preparing its meat, are risk factors.
The transmission mechanism between zoonotic reservoirs and susceptible individuals is unknown, but it is strongly suspected that direct contact with an infected armadillo poses a significant risk of developing leprosy. However, many cases reported in the U.S. have demonstrated an absence of either zoonotic exposure or person-to-person transmission outside of North America, suggesting that transmission may be happening where the infected person lives. But in many cases, the source remains an enigma.
Some people’s genetics might make them more susceptible to leprosy infections, or their immune systems are less capable of resisting the disease.
Stigma and discrimination have prevented people from seeking treatment, and as a result, “concealed” cases contribute to transmission.
How do you recognize it?
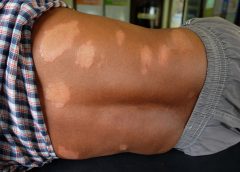
Leprosy primarily affects the skin and peripheral nervous system, causing physical deformity and desensitizing one’s ability to feel pain on affected skin.
It may begin with loss of sensation on whitish patches of skin or reddened skin. As the bacteria spread in the skin, they can cause the skin to thicken with or without nodules. If this occurs on a person’s face, it may rarely produce a smooth, attractive-appearing facial contour known as lepra bonita, or “pretty leprosy.” The disease can progress to causing eyebrow loss, enlarged nerves in the neck, nasal deformities and nerve damage.
The onset of symptoms can sometimes take as long as 20 years because the infectious bacteria have a lengthy incubation period and proliferate slowly in the human body. So presumably many people are infected long before they know that they are.
Fortunately, worldwide efforts to screen for leprosy are being enhanced thanks to organizations like the Order of Saint Lazarus, which was originally founded in the 11th century to combat leprosy, and the Armauer Hansen Research Institute, which conducts immunologic, epidemiological and translational research in Ethiopia. The nongovernmental organization Bombay Leprosy Project in India does the same.
How treatable is it?
Leprosy is not only preventable but treatable. Defying stigma and advancing early diagnosis via proactive measures are critical to the mission of controlling and eradicating it worldwide.
Notably, the World Health Organization and other agencies provide multi-drug therapy at no cost to patients.
In addition, vaccine technology to combat leprosy is in the clinical trials stage and could become available in coming years. In studies involving nine-banded armadillos, this protein-based vaccine delayed or diminished leprous nerve damage and kept bacteria at bay. Researchers believe that the vaccine can be produced in a low-cost, highly efficient manner, with the long-term prospect of eradicating leprosy.
If health care professionals, biomedical researchers and lawmakers do not markedly enhance their efforts to eliminate leprosy worldwide, the disease will continue to spread and could become a far more serious problem in areas that have been largely free of leprosy for decades.
The World Health Organization launched a plan in 2021 for achieving zero leprosy.
Robert A. Schwartz, Professor and Head of Dermatology, Rutgers New Jersey Medical School, Rutgers University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Read MoreFlesh-eating bacteria infections are on the rise in the US
By Bill Sullivan, Indiana University
Flesh-eating bacteria sounds like the premise of a bad horror movie, but it’s a growing – and potentially fatal – threat to people.
In September 2023, the Centers for Disease Control and Prevention issued a health advisory alerting doctors and public health officials of an increase in flesh-eating bacteria cases that can cause serious wound infections.
I’m a professor at the Indiana University School of Medicine, where my laboratory studies microbiology and infectious disease. Here’s why the CDC is so concerned about this deadly infection – and ways to avoid contracting it.
What does ‘flesh-eating’ mean?
There are several types of bacteria that can infect open wounds and cause a rare condition called necrotizing fasciitis. These bacteria do not merely damage the surface of the skin – they release toxins that destroy the underlying tissue, including muscles, nerves and blood vessels. Once the bacteria reach the bloodstream, they gain ready access to additional tissues and organ systems. If left untreated, necrotizing fasciitis can be fatal, sometimes within 48 hours.
The bacterial species group A Streptococcus, or group A strep, is the most common culprit behind necrotizing fasciitis. But the CDC’s latest warning points to an additional suspect, a type of bacteria called Vibrio vulnificus. There are only 150 to 200 cases of Vibrio vulnificus in the U.S. each year, but the mortality rate is high, with 1 in 5 people succumbing to the infection.
How do you catch flesh-eating bacteria?
Vibrio vulnificus primarily lives in warm seawater but can also be found in brackish water – areas where the ocean mixes with freshwater. Most infections in the U.S. occur in the warmer months, between May and October. People who swim, fish or wade in these bodies of water can contract the bacteria through an open wound or sore.
Vibrio vulnificus can also get into seafood harvested from these waters, especially shellfish like oysters. Eating such foods raw or undercooked can lead to food poisoning, and handling them while having an open wound can provide an entry point for the bacteria to cause necrotizing fasciitis. In the U.S., Vibrio vulnificus is a leading cause of seafood-associated fatality.
Why are flesh-eating bacteria infections rising?
Vibrio vulnificus is found in warm coastal waters around the world. In the U.S., this includes southern Gulf Coast states. But rising ocean temperatures due to global warming are creating new habitats for this type of bacteria, which can now be found along the East Coast as far north as New York and Connecticut. A recent study noted that Vibrio vulnificus wound infections increased eightfold between 1988 and 2018 in the eastern U.S.
Climate change is also fueling stronger hurricanes and storm surges, which have been associated with spikes in flesh-eating bacteria infection cases.
Aside from increasing water temperatures, the number of people who are most vulnerable to severe infection, including those with diabetes and those taking medications that suppress immunity, is on the rise.
What are symptoms of necrotizing fasciitis? How is it treated?
Early symptoms of an infected wound include fever, redness, intense pain or swelling at the site of injury. If you have these symptoms, seek medical attention without delay. Necrotizing fasciitis can progress quickly, producing ulcers, blisters, skin discoloration and pus.
Treating flesh-eating bacteria is a race against time. Clinicians administer antibiotics directly into the bloodstream to kill the bacteria. In many cases, damaged tissue needs to be surgically removed to stop the rapid spread of the infection. This sometimes results in amputation of affected limbs.
Researchers are concerned that an increasing number of cases are becoming impossible to treat because Vibrio vulnificus has evolved resistance to certain antibiotics.
How do I protect myself?
The CDC offers several recommendations to help prevent infection.
People who have a fresh cut, including a new piercing or tattoo, are advised to stay out of water that could be home to Vibrio vulnificus. Otherwise, the wound should be completely covered with a waterproof bandage.
People with an open wound should also avoid handling raw seafood or fish. Wounds that occur while fishing, preparing seafood or swimming should be washed immediately and thoroughly with soap and water.
Anyone can contract necrotizing fasciitis, but people with weakened immune systems are most susceptible to severe disease. This includes people taking immunosuppressive medications or those who have pre-existing conditions such as liver disease, cancer, HIV or diabetes.
It is important to bear in mind that necrotizing fasciitis presently remains very rare. But given its severity, it is beneficial to stay informed.
Bill Sullivan, Professor of Pharmacology & Toxicology, Indiana University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
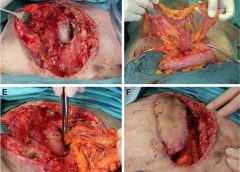
Read MoreOmentum flap as a salvage procedure in deep sternal wound infection
Introduction: Deep sternal wound infections (DSWIs) are rare but devastating complication after median sternotomy following cardiac surgery. Especially in the presence of artificial material or inadequate preliminary muscle flaps, the pedicled omentum flap is due to its immunological properties, the predetermined flap in salvage procedures. (more…)
Read MoreScientists Seek People with Primary Progressive MS and Other Forms of MS to Study Gut Bacteria
Investigators at the University of California in San Francisco are recruiting people with MS for an international study of the gut microbiome – the population of bacteria in the gut – in MS. They are seeking people with primary progressive MS nationwide (there is no need for onsite visits), as well as people with any other type of MS who can make a one-time visit to San Francisco, New York, Boston or Pittsburgh. The overall purpose of these studies is to investigate the potential role of gut bacteria in MS.
Scientists Focus on Gut Flora for Future Treatments of Autoimmune Diseases
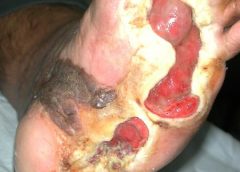
Read MoreGene Therapy for Non-Healing Diabetic Foot Ulcers Starts Phase III Trial
Safety and Efficacy Study of VM202 in the Treatment of Chronic Non-Healing Foot Ulcers. This study will assess the safety and efficacy of using gene therapy via intramuscular injections of the calf for patients with chronic non-healing foot ulcers.
The first patient has been dosed in a Phase III trial assessing ViroMed’s VM202, the first pivotal study of a gene therapy indicated for patients with nonhealing diabetic foot ulcers (NHU) and concomitant peripheral artery disease (PAD).
The Phase III trial (NCT02563522) is a double-blind, placebo-controlled, multicenter study designed to evaluate VM202 for safety and efficacy in 300 adults with a diabetic foot ulcer and concomitant PAD. Two hundred patients will be randomized to VM202 and the other 100 to placebo, ViroMed’s U.S. division VM BioPharma said yesterday. (more…)
Read MoreA new way of healing wounds in the future discovered by scientists
Scientists at Ohio State University have developed a new method that has the capability of changing the body’s existing cells into new cells to promote healing. The method, called Tissue Nanotransfection (TNT), reprograms cells through a device that uses nanotechnology. The way it would work: First, doctors would apply a light electrical stimulation to the surface of the skin. They would then place a small chip about the size of a cuff link onto the site of the wound. In less than a second, this chip would deliver reprogramming factors (pre-programmed DNA or RNA) non-invasively into living skin cells via a high-intensity, focused electric field, converting them into whatever type of cells a scientist or doctor may choose. (more…)
Read MoreAntibiotic use in pressure injury infections
Antibiotic overuse contributes to the problems of antibiotic resistance and healthcare acquired infections, such as Clostridium difficile. Antibiotic stewardship programs improve patient outcomes, reduce antimicrobial resistance, and save money. These programs are designed to ensure patients receive the right antibiotic, at the right dose, at the right time, and for the right duration. (more…)
Read MoreBetter Skin Grafts – take only one layer
Research shows that a skin-graft harvesting system aids chronic wound recovery and reduces care costs by accelerating the healing process.
More than six million cases of chronic wounds cost $20 billion each year in the United States. Diabetic ulcers, pressure sores, surgical site wounds, and traumatic injuries to high-risk patients account for most wounds that won’t heal. (more…)
Read More