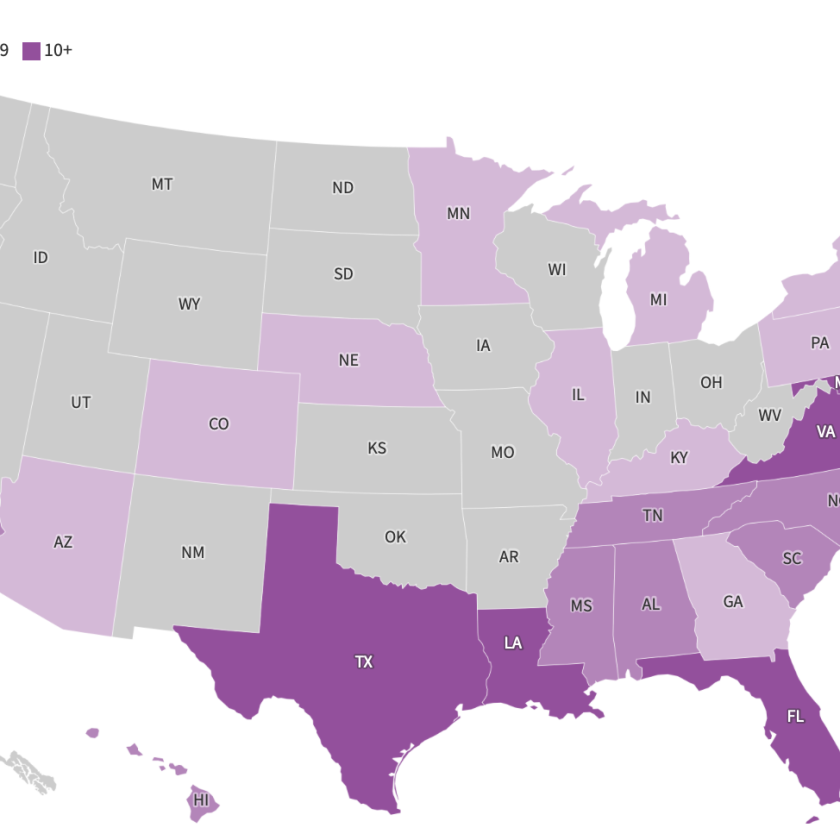
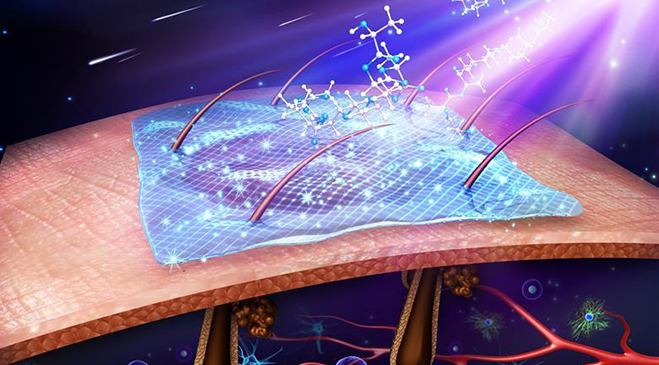
Scientists at Ohio State University have developed a new method that has the capability of changing the body’s existing cells into new cells to promote healing. The method, called Tissue Nanotransfection (TNT), reprograms cells through a device that uses nanotechnology. The way it would work: First, doctors would apply a light electrical stimulation to the surface of the skin. They would then place a small chip about the size of a cuff link onto the site of the wound. In less than a second, this chip would deliver reprogramming factors (pre-programmed DNA or RNA) non-invasively into living skin cells via a high-intensity, focused electric field, converting them into whatever type of cells a scientist or doctor may choose.
This technique has previously been tested in mouse models to salvage dead tissue and promote limb re-growth. TNT is different from previous technologies in that it is a benign, instantaneous and dose-controlled re-programming factor delivery method at the single cell level. Researchers expect the technology to be approved for human trials within a year. While it still needs to be tested for safety and efficacy in humans, this technology may ultimately have implications for cell reprogramming in conditions such as stroke, Alzheimer’s and Parkinson’s.
Topical tissue nano-transfection mediates non-viral stroma reprogramming and rescue.
Although cellular therapies represent a promising strategy for a number of conditions, current approaches face major translational hurdles, including limited cell sources and the need for cumbersome pre-processing steps (for example, isolation, induced pluripotency)1, 2, 3, 4, 5, 6. In vivocell reprogramming has the potential to enable more-effective cell-based therapies by using readily available cell sources (for example, fibroblasts) and circumventing the need for ex vivo pre-processing7, 8. Existing reprogramming methodologies, however, are fraught with caveats, including a heavy reliance on viral transfection9, 10. Moreover, capsid size constraints and/or the stochastic nature of status quo approaches (viral and non-viral) pose additional limitations, thus highlighting the need for safer and more deterministic in vivo reprogramming methods11, 12. Here, we report a novel yet simple-to-implement non-viral approach to topically reprogram tissues through a nanochannelled device validated with well-established and newly developed reprogramming models of induced neurons and endothelium, respectively. We demonstrate the simplicity and utility of this approach by rescuing necrotizing tissues and whole limbs using two murine models of injury-induced ischaemia.
Recent advances in nuclear reprogramming in vivo have opened up the possibility for the development of ‘on-site’, patient-specific, cell-based therapies. We have developed a novel yet simple to implement non-viral approach to topically and controllably deliver reprogramming factors to tissues through a nano-channelled device (Fig. 1). This tissue nano-transfection (TNT) approach allows direct cytosolic delivery of reprogramming factors by applying a highly intense and focused electric field through arrayed nano-channels11,13, which benignly nanoporates the juxtaposing tissue cell membranes and electrophoretically drives reprogramming factors into the cells (Fig. 1a–d). Detailed information regarding the TNT system fabrication process and simulation results is provided in Supplementary Figs 1 and 2. In contrast to current in vivo transfection technologies (for example, viruses and conventional tissue bulk electroporation (BEP)), in which gene delivery is highly stochastic in nature and could lead to adverse side effects (such as inflammatory response and cell death)14, nanochannel-based transfection enables more focused (Fig. 1b,c) and ample (Fig. 1d) reprogramming factor delivery at the single-cell level, thus making this a powerful tool for deterministic in vivo gene transfection and reprogramming 11,13.
read the study on nature.com.