PolarityTE (TM) Regenerates Full-Thickness Hair-Bearing Skin in Burns and Wounds Using Their Revolutionary Platform Technology. First ever known successful regeneration of full-thickness skin and hair; Company poised to initiate human trial in the third quarter of 2017; Management to host conference call Thursday, June 8th at 4:30pm ET.
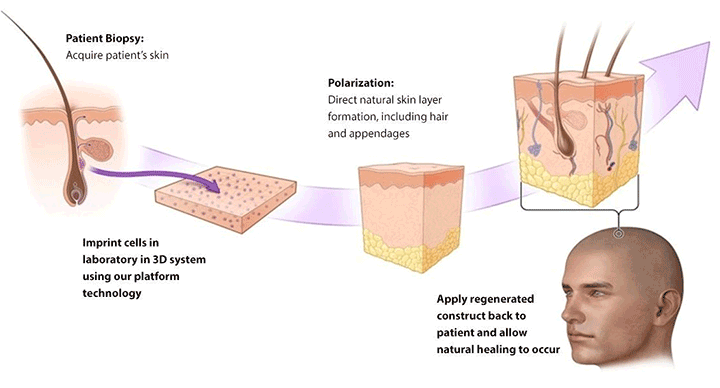
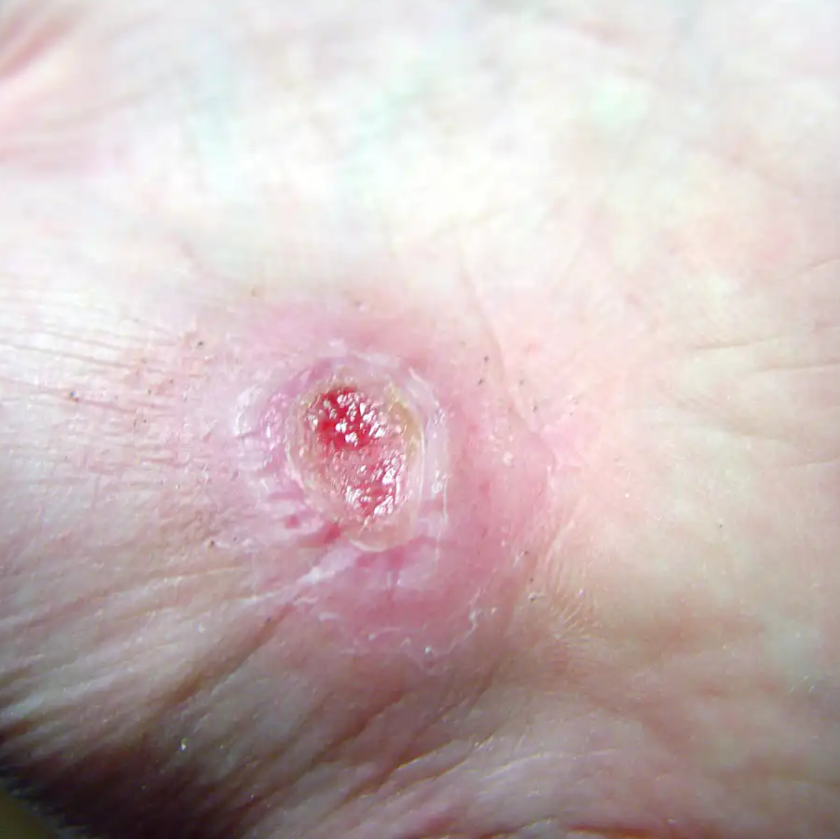
Salt Lake City, UT — (Marketwired) — 06/08/17 — PolarityTE™, Inc. (NASDAQ: COOL) today announced pre-clinical results demonstrating that the Company’s lead product, SkinTE™, regenerated full-thickness, organized skin and hair follicles in third degree burn wounds. The findings represent the first known successful regeneration of skin and hair in full-thickness swine wound models, the standard animal model for human skin. The Company expects to initiate a human clinical trial evaluating the autologous homologous SkinTE™ construct in the third quarter of 2017.
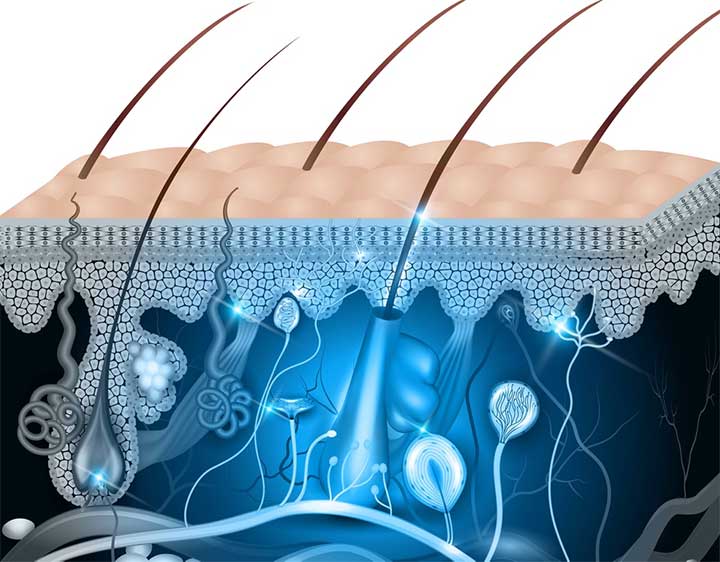
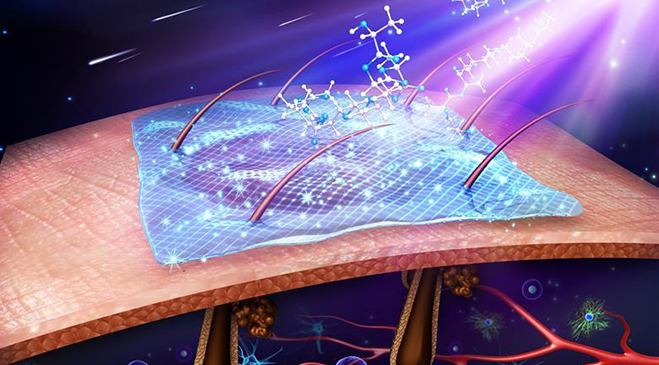
In pre-clinical models of full-thickness burns and wounds, SkinTE™ demonstrated scar-less healing, hair follicle growth, immediate complete wound coverage, and the progressive regeneration of all skin layers including epidermis, dermis and hypodermal layers (www.polarityte.com/products/skinte). The SkinTE™ product, which utilizes the subject’s own skin, is prepared and used to treat the wound in less than 24 hours.
“These findings using SkinTE™ demonstrate an entirely new and pragmatic system whereby Polarity has used autologous tissue to regenerate full-thickness skin, hair follicles and appendages for the treatment of burns and wounds. This is a first for regenerative medicine and validation of the Polarity regenerative tissue platforms in pre-clinical studies across a variety of species,” said Denver M. Lough, M.D., Ph.D., Chairman and Chief Executive Officer of PolarityTE™. “We look forward to putting the SkinTE™ construct on human patients this year and releasing more data on our unique Polarity platform technology and pipeline of patient-specific constructs such as OsteoTE™ and CartTE™ shortly.”
Swine models of burns and wounds are known to be predictive of results found in humans due to the unique similarities between swine and human skin. Of note, it is believed that swine skin may be more difficult to regenerate with all layers and appendages (hair and glands), as was done in the studies by PolarityTE™, suggesting that the results of these studies may predict similar efficacy in human patients when clinical trials begin later this year.
“Our revolutionary approach to a new form of regenerative healing offers hope to both burn and wound patients, as well as medical providers who have not seen a significant advance in skin regeneration since the 1980s,” said Professor Stephen Milner, MD, DDS, DSc, FRCS(Ed), FACS, Chief Clinical Officer of PolarityTE and prior Director of the Johns Hopkins Burn Centers. “The efficacy and utility of current approaches to burn and wound care are currently severely limited, and leave a lasting effect on patients who suffer with scarring, disfigurement or even death. I believe that this astonishing physician-led team and unique technology platform, which uses the patient’s own tissues, has the potential to revolutionize regenerative medicine and be the foundation for a new paradigm of treatment and outcomes.”
Conference Call and Webcast Information
PolarityTE will host a conference call and live webcast for investors, analysts and other interested parties on Thursday, June 8, 2017 at 4:30pm ET to discuss today’s announcement.
To participate in the conference call, dial 877-407-8831 (U.S.) or 201-493-6736 (International). The live webcast will be accessible on the Events page of the Investors section of PolarityTE’s website, www.polarityte.com, or available here. The webcast will be archived for 180 days.
About SkinTE and the PolarityTE Platform
SkinTE is the Company’s lead product in development for skin regeneration. Its investigational platform and the Company’s namesake, PolarityTE, is being developed to simplify regeneration and allow tissue and cellular elements to function naturally. Using our revolutionary platform, we seek to utilize cell and tissue polarity in order to create a spectrum of uniquely functional tissues in a way that mirrors the natural development of the human body. Our goal is to apply the platform across all cells, tissues and composite structures, transforming regenerative medicine into what has been envisioned since its inception.
About PolarityTE™
PolarityTE™, Inc. is a regenerative medicine company positioned to be the first to successfully regenerate human skin. The Company’s novel regenerative medicine and tissue engineering platform was developed and patented by chairman and chief executive officer, Denver Lough M.D., Ph.D. This radical and proprietary technology employs a patient’s own cells for the healing of full-thickness, functionally-polarized tissues. If clinically successful, the PolarityTE™ platform will provide medical professionals with a truly new paradigm in wound healing and reconstructive surgery by utilizing a patient’s own tissue substrates for the regeneration of skin, bone, muscle, cartilage, fat, blood vessels and nerves. The PolarityTE™ platform leverages natural and biologically-sound principles which are readily adaptable to a wide spectrum of organ and tissue systems. This revolutionary technology, paired with the Company’s world-renowned clinical advisory board, position PolarityTE™ to drastically change the field and future of translational regenerative medicine. More information can be found online at www.polarityte.com.
Forward Looking Statements
Certain statements contained in this release are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward looking statements contained in this release relate to, among other things, the success of the Company’s lead product, SkinTE™, the results of future clinical trials and the Company’s ability to initiate human clinical trials or the success thereof. They are generally identified by words such as “believes,” “may,” “expects,” “anticipates,” “should'” and similar expressions. Readers should not place undue reliance on such forward-looking statements, which are based upon the Company’s beliefs and assumptions as of the date of this release. The Company’s actual results could differ materially due to risk factors and other items described in more detail in the “Risk Factors” section of the Company’s Annual Reports and other filings with the SEC (copies of which may be obtained at www.sec.gov). Subsequent events and developments may cause these forward-looking statements to change. The Company specifically disclaims any obligation or intention to update or revise these forward-looking statements as a result of changed events or circumstances that occur after the date of this release, except as required by applicable law.
Source: PolarityTE, Inc.