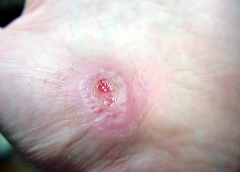
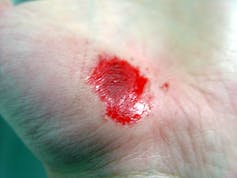
Search Results for: wound
Oestrogen offers wound healing properties
Mission possible: Getting Medicare reimbursement for wound care in acute-care settings
By Susan Reinach-Lannan, BSOM
In the current healthcare environment, wound care practitioners need to capitalize on all available reimbursement avenues for care delivery and wound care supplies and dressings. And when it comes to reimbursement, there’s one constant: The rules change constantly. Whether these changes always benefit the patient is questionable. Nowhere is this more evident than in acute-care settings. Clinicians constantly are challenged to make sure their patient-care decisions comply with current Medicare reimbursement guidelines. (And if you’re not sure about today’s guidelines, be prepared for the guidelines to change tomorrow.) (more…)
Read MoreGet positive results with negative-pressure wound therapy
By Ronald Rock, MSN, RN, ACNS-BC
Complex wound failures are costly and time-consuming. They increase length of stay and contribute to morbidity and mortality in surgical patients. Negative-pressure wound therapy (NPWT)—a common adjunct to wound-care therapy—is used to accelerate wound healing in all fields of surgery. Using a vacuum device and wound-packing material, it applies subatmospheric pressure to complex wounds.
But NPWT alone doesn’t ensure adequate wound healing. Many physiologic factors—including infection, excessive moisture, nutrition, and medications—influence wound-healing success. Failure to account for these factors or improper application of NPWT can limit patient outcomes and cause debilitating complications.
For clinicians, applying and establishing an airtight seal on a complex wound is among the most dreaded, time-consuming, and challenging NPWT-related tasks. Simply applying NPWT material under layers of transparent drape may delay wound healing or exacerbate the wound. This article provides tips on safe application of NPWT to enhance the outcomes of patients with complex wounds.
Consider wound location
Wounds on the body’s anterior surfaces are less susceptible to the forces of pressure, friction, and shear than those on posterior and lateral surfaces. Posterior and lateral wounds commonly require posterior offloading or repositioning the patient in bed to reduce or eliminate direct pressure. This can be done with judicious and frequent patient turning using a specialty bed or support surface.
Bridge a posterior or lateral wound to an anterior surface by placing the drainage collection tubing to a nonpressure-bearing surface away from the wound. Bridging keeps the tubing from exerting pressure on intact skin and decreases the risk of a pressure ulcer. To create the bridge, cut foam into a single spiral of 0.5 to 1 cm, or if using gauze, fold gauze into 8 single layers.
Place the spiraled foam or gauze layers onto the drape, ensure the bridge is wider than the collection tubing disc, and secure it with an additional drape. Next, apply the NPWT collection tubing on the end of the bridge away from the wound. A wide bridge under the collection tubing disc will minimize the potential for periwound breakdown when negative pressure is initiated. You may modify this spiraling technique by varying the width of the foam to fill undermining and wounds of irregular configuration and depth.
Protect the periwound
An intact periwound may break down from exposure to moisture, injury from repetitive removal of a transparent drape, or NPWT material coming in contact with skin. Skin protection is critical in preventing additional breakdown stemming from contact with potentially damaging material.
Transparent drapes are designed to permit transmission of moisture vapor and oxygen. Avoid using multiple layers of transparent drapes to secure dressings over intact skin, as this can decrease the transmission of moisture vapor and oxygen, which in turn may increase the risk of fungal infection, maceration, and loss of an intact seal.
Periwound maceration also may indicate increased wound exudate, requiring an increase in negative pressure. Conversely, an ecchymotic periwound may indicate excessively high negative pressures. If either occurs, assess the need to adjust negative pressure and intervene accordingly. Reassess NPWT effectiveness with subsequent dressing changes.
Apply a protective liquid skin barrier to the periwound and adjacent healthy tissue to help protect the skin surface from body fluids. The skin barrier also helps prevent stripping of fragile skin by minimizing shear forces from repetitive or forceful removal of transparent drapes. Excessive moisture can be absorbed by using a light dusting of ostomy powder sealed with a skin barrier. A “window pane” of transparent drape or hydrocolloid dressing around the wound also can protect surface tissue from contactwith NPWT material and prevent maceration.
Avoid creating rolled wound edges
In the best-case scenario, epithelial tissue at the wound edge is attached to the wound bed and migrates across healthy granulation tissue, causing the wound to contract and finally close. With deep wound environments that lack moisture or healthy granulation tissue, the wound edges may roll downward and epibole may develop. Epibole is premature closure of the wound edges, which prevents epithelialization and wound closure when it comes in contact with a deeper wound bed. (See Picturing epibole by clicking the PDF icon above.)
Materials used in NPWT are primarily air-filled. Applying negative pressure causes air removal, leading to wound contraction by pulling on the wound edges—an action called macrostrain. Without sufficient NPWT material in the wound, macrostrain can cause the wound to contract downward and the wound edges to roll.
Ensure that enough NPWT material has been applied into the wound to enhance wound-edge approximation and avoid creating a potential defect as the wound heals. Before NPWT begins, material should be raised 1 to 2 cm above the intact skin. Additional material may be needed with subsequent changes if the NPWT material compresses below the periwound. The amount of NPWT material needed to remain above the periwound once NPWT starts varies with the amount of material compressed and the wound depth.
Reduce the infection risk
To some degree, all wounds are contaminated. Usually, the body’s immunologic response is able to clear bacterial organisms and wound healing isn’t delayed. But a patient who has an infection of a complex wound needs additional support.
Systemic antibiotics alone aren’t enough because they’re selective for specific organisms and don’t reach therapeutic levels in the wound bed. In contrast, topical antimicrobial adjuncts, such as controlled-release ionic silver, provide broad-spectrum antimicrobial coverage against fungi, viruses, yeasts, and gram-negative and gram-positive bacteria, including methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci.
Consider using controlled-release ionic silver for a wound known to be infected or at risk for infection due to its location or potential urine or fecal contamination. To be bactericidal, ionic silver must be in concentrations of at least 20 parts per million; also, it must be kept moist and must come in direct contact with infected wound bed. At lower concentrations, organisms may develop resistance. Ionic silver has no known resistance or contraindications. Dressings using it come in several forms, including a hydrogel sheet, perforated sheet, cavity version, and semiliquid hydrogel. Be sure the form you choose doesn’t occlude the NPWT material and compromise therapy. (See NPWT for a patient with necrotizing fasciitis by clicking the PDF icon above.)
View: NPWT
Obtain a negative-pressure environment
One of the most daunting aspects of NPWT is obtaining and maintaining a good seal—in other words, avoiding the dreaded leak. Preventive skin measures may contribute to a poor seal; skin-care products containing glycerin, surfactant, or dimethicone may prevent adequate adhesion of NPWT drapes. Body oil, sweat, and hair may need to be minimized or removed.
To avoid leaks, don’t overlook the obvious—loose connections, a loose drainage collection canister, exposed NPWT material, and skinfolds extending beyond the transparent drape. Tincture of benzoin (with or without a thin hydrocolloid dressing) increases tackiness to enhance the adhesive property of a transparent drape on the diaphoretic patient and on hard-to-drape areas, such as the perineum. But be sure to use tincture of benzoin with discretion, as it may remove fragile periwound tissue when the dressing is removed.
Ostomy paste products can serve as effective filler. These pliable products can be spread into position to obtain a secure seal under the transparent drape in hard-to-seal areas, such as the perineum. Pastes remain flexible and can be removed without residue. Temporarily increasing NPWT pressure to a higher setting may help locate a subtle leak or provide enough negative pressure to self-seal the leak. Once the leak resolves, remember to return the pressure to the ordered setting.
Knowledge optimizes healing
It’s important to be aware of potential complications of NPWT (See Take care with NPWT by clicking on the PDF icon above). However, when applied correctly, NPWT is an effective option for managing complex wounds. Recognizing and managing potential complications at the wound site, ensuring periwound protection, minimizing epibole formation, and preventing wound infection can result in a better-prepared wound bed and promote optimal healing.
View: NPWT case study
Selected references
Baranoski S, Ayello EA. (2012). Wound Care Essentials: Practice Principles. 3rd ed. Springhouse, PA; Lippincott Williams & Wilkins.
Bovill E, Banwell PE, Teot L, et al. Topical negative pressure wound therapy: a review of its role and guidelines for its use in the management of acute wounds. Int Wound J. 2008;5:511-529.
Sussman C, Bates-Jensen B. Wound Care: A Collaborative Practice Manual for Health Professionals. 4th ed. Baltimore, MD; Lippincott Williams & Wilkins; 2011.
Ronald Rock is an Adult Health Clinical Nurse Specialist in the Digestive Disease Institute at the Cleveland Clinic in Cleveland, Ohio.
Read MoreProviding wound care in the home: An option to explore
By Connie Johnson, RN, BSN, WCC, LLE, DAPWCA
Jim, a 52-year-old patient with colon cancer, received a new ostomy. He needed a custom fit for his appliance, which took 10 days. During this time, trying to obtain a good seal and treat the peristomal area wasn’t easy. Despite my best efforts, Jim’s skin was denuded from contact with stool. Although he was in great discomfort, he wanted to wait until my next visit to tell me about the problem. Fortunately, his wife was worried and contacted me directly.
Jim lives in a neighborhood with a low crime rate, so I’m able to see him within
a few hours of his wife’s call, even though it’s late at night. As it turns out, I make
extra visits to help him manage his stoma until the customized appliance is ready. As with any home care situation, I’m ready to do my best for my patient.
Many home-care patients like Jim benefit from the interventions of a wound care clinician (WCC). More than one-third of all home-care admissions are wound related, and home wound care has become one of the fastest growing needs and skills in home-care services. So if you’re a WCC, you may want to consider home care as a practice option.
Delivering wound care in the home differs dramatically from delivering it in the hospital. Given the complexity of wound care and the multiple factors that affect healing, home wound care is a challenge. Some patients have chronic conditions, such as diabetes or wounds or open sores that don’t heal easily. In other cases, the patient or caregiver is unable to change dressings. That’s where the WCC comes in.
Special needs of home-care patients
Like other patients across the continuum of care, home-care wound patients require accurate and thorough wound assessment, as well as documentation that provides information about wound status and aids development of a plan that supports healing.
Of course, the plan of care must address the whole patient, not just the “hole” in the patient. The WCC must take into account comorbidities, individual wound-care requirements, assistance the patient may need due to physical or mental deficits, and nutritional support. Additional factors that affect wound-care strategies include wound characteristics, family support, and insurance guidelines and reimbursement.
Role of the WCC
The WCC’s role in home care includes providing clinical expertise, working with other healthcare team members, and providing education.
- The WCC provides clinical expertise regarding wound and ostomy care to ensure delivery of the highest quality of care. This expertise helps reduce the need for readmissions to the emergency department (ED) for wound-related complications. The WCC also plays a vital role in product awareness, formu-lary development, and maintenance of cost-effective, evidence-based practice in the agency.
- Working with other healthcare team members, the WCC serves as patient advocate, strengthening the relationship between patient and healthcare team members while promoting care coordination to help the patient achieve goals. Effective communication with the patient’s primary care provider is essential to delivering the best-quality, research-based wound care. A tool for strengthening such communication is the SBAR (Situation-Background-Assessment-Recommendation) technique. SBAR structures conversations so all parties provide complete yet concise information. (See SBAR wound and skin provider communication record by clicking the PDF icon above).
- The WCC educates patients and family members about wound healing, dressing applications, and other interventions. Teaching families allows them to be involved in the patient’s care and start to take ownership of it. The WCC also educates home health aides, who can play a vital role in preventing such problems as pressure ulcers and may be responsible for ensuring staff members are aware of the products, procedures, and dressings available.
Challenges of home care
If you’re a WCC and considering home care as a career option, know that practicing in the home can be a real eye opener. For starters, consider geography. Shortly after I started as a wound care nurse/consultant in home care, I was visiting patients all over New Jersey, some days driving 200 miles. As I quickly discovered, once you enter the home, don’t assume you’ll simply change a dressing and then be on your way. Instead, you may find you are, in essence, the family case manager who’s expected to “fix everything.” This role requires equal doses of planning and creativity.
What’s more, expect to do some improvising. In acute-care settings, all the supplies you may need to prevent infection—gowns, gloves, masks—usually are within arm’s reach. But in home care, these supplies may be absent, meaning you’ll need to set up the cleanest environment you can under the circumstances. That might mean using disposable drapes and dressings. Be sure to carry large amounts of hand sanitizer.
Dressing selection is perhaps the biggest challenge in home wound care because
it involves not just wound-specific issues but financial and practical considerations. The ideal dressing in the home is one that needs to be changed only every other day, at most. Evidence shows it’s not practical to try to change dressings two or three times daily at home unless the family is providing care.
Develop a checklist
Because the home environment may lack all the resources you need, remembering everything you need to do before you leave the patient’s home may be challenging. To help keep things on track, develop a checklist of reminders that covers these points:
- Have necessary medical appointments been arranged? Does the patient have transportation to appointments?
- Are there sufficient supplies in the home?
- Is there enough medicine? If not, who will pick up the medicine?
- Are consults needed, such as social worker or physical therapist?
- Who will help with any activities of daily living that the patient is unable to do?
- Does the patient with diabetes have a glucometer?
Hours and safety concerns
Typical home wound-care hours are 8:30 a.m. to 4:30 p.m. But realistically, expect variations. For instance, as you’re about to leave, the patient might say, “My wife isn’t feeling well. Could you take her blood pressure?” This means you’ll stay a little longer.
When planning home visits, be aware of safety concerns. If visiting after hours could put you in danger, it’s safer to instruct the patient to call an ambulance and go to the local ED.
Reimbursement
Reimbursement is an important factor in wound care in the home. To be eligible for home care through Medicare, patients must be homebound—meaning they don’t routinely travel to run errands or visit or they’re not able to obtain or receive needed medical services. (With private insurance and workers’ compensation, eligibility requirements may be less restrictive.)
Know that a Medicare patient receives home care as an “episode.” Episodes are 60-day periods; within each 60-day episode, a $592 cap is allotted should a patient require supplies for wound or ostomy care needs. Except for negative-pressure wound therapy, a home care agency can’t bill Medicare for products used; instead, the home-care agency is responsible for the cost of all topical wound-care products and dressings. Agencies may keep patients on service even if they exceed the allowed amount, although patients reaching maximum benefits commonly are discharged from service. Home-care agencies have no choice but to discharge Medicare patients they find aren’t truly homebound.
Also, be aware that Medicare views home health service as an interim service. When a patient is no longer making progress, Medicare expects that the family will provide the patient’s care or the patient will enter a skilled care facility. So it’s important to work hard to obtain good outcomes—not just for the patient but to maintain Medicare reimbursement. Like many private insurance companies, Medicare reimbursement is based on pay for performance; if an agency doesn’t deliver optimal outcomes, it receives lower reimbursement, increasing its financial burden.
A worthwhile option
WCCs use their knowledge and clinical expertise to improve patient outcomes and teach patients, families, and other healthcare team members. They also give the agency recommendations for care and supplies that are evidence based and reflect current best practices in wound care. Accomplishing these goals in a timely fashion under various constraints can be challenging. But if you choose to work in the home, try to keep a smile on your face and joy in your voice for each patient and family. If you like challenges and want a job where you can apply your creativity and function independently, becoming a home-care WCC might be the right choice for you.
Connie Johnson provides wound care in the home and in acute-care settings.
Read MoreWound Care Meta-Review Draws Firm Conclusions
Protein may be key to psoriasis, wound care
Discovery Promises Unique Medicine for Treatment of Chronic and Diabetic Wounds
Caring for chronic wounds: A knowledge update
By: Patricia A. Slachta, PhD, RN, ACNS-BC, CWOCN
Wound care has come a long way in just a few decades. With our expanded knowledge of wound healing and recent advances in treatment, we’re now able to assess wounds more accurately, recognize wound-related problems sooner, provide better interventions, and reduce morbidity.
To bring you up to date on current evidence-based wound management, this article focuses on assessing patients with chronic wounds, optimizing wound healing with effective wound-bed preparation, and selecting an appropriate dressing.
Wound chronicity and cause
Developing an appropriate plan of care hinges on conducting a thorough, accurate evaluation of both the patient and the wound. The first step is to determine whether the wound is acute or chronic.
• A chronic wound is one that fails to heal within a reasonable time—usually
3 months.
• An acute wound heals more quickly, causing minimal functional loss in the part of the body with the wound.
Identifying the cause of the wound also is essential. If the wound etiology is unknown, explore the patient’s medical history (including medication history) for clues to possible causes. Also review the patient’s history for conditions that could impede wound healing. (See What factors hamper healing? by clicking the PDF icon above)
Other important aspects of assessment include evaluating the patient’s nutritional status, quantifying the level of pain (if present), and gauging the patient’s self-care abilities.
General physical appearance
Conduct a general head-to-toe physical examination, focusing on the patient’s height, weight, and skin characteristics.
Height, weight, and weight trend
On admission, the patient’s height and weight should be measured to ensure appropriate nutritional and pharmacologic management. After a weight gain or loss, various factors may complicate wound healing. For instance, involuntary weight loss and protein-energy malnutrition may occur in both acute-care and long-term-care patients.
Especially note trends in your patient’s weight. For a long-term-care patient, a 5% weight loss over 30 days or a 10% loss over 180 days is considered involuntary. Arrange for a nutritional consult for any patient with an involuntary weight loss, as adequate nutrition is essential for general well-being and wound healing. (See A wound on the mend by clicking the PDF icon above.)
Skin color
Evaluate the patient’s skin color in light of ethnic background. If you note erythema—especially on a pressure point over a bony prominence—examine this area carefully for nonblanching erythema. Keep in mind that darkly pigmented skin doesn’t show such erythema and subsequent blanching, yet the patient may still be in jeopardy. So in dark-skinned patients, check for differences in skin color, temperature, or firmness compared to adjacent tissue; these differences may signify skin compromise.
Skin texture and turgor
Generally, healthy skin feels smooth and firm and has an even surface and good turgor (elasticity). To test turgor, gently grasp and pull up a fold of skin on a site such as the anterior chest below the clavicle. Does the skin return to place almost immediately after you release it, or does it stand up (“tent”)? Tenting indicates dehydration. But keep in mind that skin loses elasticity with age, so elderly patients normally have decreased turgor.
Skin temperature
With normal circulatory status, the skin is warm and its temperature is similar bilaterally. Areas of increased warmth or coolness suggest infection or compromised circulation. Be sure to check the temperature of skin surrounding the wound.
Wound assessment
Proper wound assessment can significantly influence patient outcome. Measure the wound carefully and document the condition of the wound bed. Remember that accurate descriptions are essential for guiding ongoing wound care. Repeat wound measurement and wound-bed assessment at least weekly, after the wound bed has been cleaned and debrided.
Keep in mind that assessing a chronic wound can be challenging. Wounds commonly have irregular shapes that can change quickly. Also, the multiple clinicians caring for the same patient may each describe the wound a bit differently.
Wound location
Note the precise anatomic location of the wound, as this can influence the wound care plan. A venous ulcer on the lower leg, for instance, requires different care than an arterial ulcer in the same site or a pressure ulcer on the ischium.
Circumference and depth
Use a paper or plastic measuring device to measure wound circumference and depth in centimeters (cm) or millimeters (mm). To promote accurate assessment of healing, be sure to use the same reference points each time you measure the wound.
You can use several methods to measure circumference. The most commonly used method of measurement is done in the head to toe direction. Measure the wound at its greatest length in that direction & measure the width at a 90 degree angle, at the widest point of the wound. Then multiply these two measurements (greatest length x greatest width) to obtain the total wound area. Although such linear measurements are imprecise, they yield gross information relative to wound healing when repeated over time.
Classify wound depth as partial thickness or full thickness.
• Partial-thickness wounds are limited to the skin layers and don’t penetrate the dermis. They usually heal by reepithelialization, in which epidermal cells regenerate and cover the wound. Abrasions, lacerations, and blisters are examples of partial-thickness wounds.
• Full-thickness wounds involve tissue loss below the dermis.
(Note: Pressure ulcers usually are classified by a four-stage system and diabetic foot ulcers by a grading system. Both systems are beyond this article’s scope.)
Measure and record wound depth based on the deepest area of tissue loss. To measure depth, gently place an appropriate device (such as a foam-tipped applicator) vertically in the deepest part of the wound, and mark the applicator at the patient’s skin level. Then measure from the end of the applicator to the mark to obtain depth.
Surrounding skin and tissue
Inspect for and document any erythema, edema, or ecchymosis within 4 cm of the wound edges, and reevaluate for these signs frequently. Because compromised skin near the wound is at risk for breakdown, preventive measures may be necessary.
Appearance of wound-bed tissue
Document viable tissue in the wound bed as granulation, epithelial, muscle, or subcutaneous tissue. Granulation tissue is connective tissue containing multiple small blood vessels, which aid rapid healing of the wound bed; appearing red or pink, it commonly looks shiny and granular. Epithelial tissue consists of regenerated epidermal cells across the wound bed; it may be shiny and silvery.
Check for nonviable tissue (also called necrotic, slough, or fibrin slough tissue), which may impede wound healing. It may vary in color from black or tan to yellow, and may adhere firmly or loosely to the wound bed. (See Picturing a necrotic wound by clicking the PDF icon above.)
Be sure to document the range of colors visible throughout the wound. Identify the color that covers the largest percentage of the wound bed. This color—and its significance—guide dressing selection.
Wound exudate
Document the amount, color, and odor of exudate (drainage) in the wound. Exudate with high protease levels and low growth factor levels may impede healing.
If the wound is covered by an occlusive dressing, assess exudate after the wound has been cleaned. Describe the amount of exudate as none, minimal, moderate, or heavy.
Describe exudate color as serous, serosanguineous, sanguineous, or purulent. Serous exudate is clear and watery, with no debris or blood present. Serosanguineous exudate is clear, watery, and tinged pink or pale red, denoting presence of blood. Sanguineous exudate is bloody, indicating active bleeding. Purulent exudate may range from yellow to green to brown or tan.
Describe wound odor as absent, faint, moderate, or strong. Note whether the odor is present only during dressing removal, if it disappears after the dressing is discarded, or if it permeates the room.
Wound edges
Wound edges indicate the epithelialization trend and suggest the possible cause and chronicity of the wound. The edges should attach to the wound bed. Edges that are rolled (a condition called epibole) indicate a chronic wound, in which epithelial cells are unable to adhere to a moist, healthy wound bed and can’t migrate across and resurface the wound.
Undermining and tracts
Gently probe around the wound edges and in the wound bed to check for undermining and tracts. Undermining, which may occur around the edges, presents as a space between the intact skin and wound bed (resembling a roof over part of the wound). It commonly results from shear forces in conjunction with sustained pressure. A tract, or tunnel, is a channel extending from one part of the wound through subcutaneous tissue or muscle to another part.
Measure the depth of a tract or undermining by inserting an appropriate device into the wound as far as it will go without forcing it. Then mark the skin on the outside where you can see or feel the applicator tip. Document your findings based on a clock face, with 12 o’clock representing the patient’s head and 6 o’clock denoting the feet. For instance, you might note “2.0-cm undermining from 7:00 to 9:00 position.”
Pain level
Ask the patient to quantify the level of pain caused by the wound, using the pain scale designated by your facility. Find out which pain-management techniques have relieved your patient’s pain in the past; as appropriate, incorporate these into a pain-management plan. Reevaluate the patient’s pain level regularly.
Wound-bed preparation
An evolving science, wound-bed preparation is crucial for minimizing or removing barriers to healing. The goal is to minimize factors that impair healing and maximize the effects of wound care. The key elements of wound-bed preparation are controlling bioburden and maintaining moisture balance. (For online resources on wound-bed preparation and other wound-care topics, see Where to get more information by clicking the PDF icon above.)
Controlling bioburden
Necrotic tissue and exudate harbor bacteria. A wound’s bioburden—the number of contaminating microbes—contributes to poor healing. All chronic wounds are considered contaminated or colonized, but not necessarily infected. In a colonized wound, healing is impeded as bacteria compete for nutrients; also, bacteria have harmful byproducts. To control bioburden, the wound must be cleaned and necrotic tissue must be debrided.
Cleaning the wound. Clean the wound before assessing it and applying a dressing. Use a noncytotoxic agent (typically, potable water, normal saline irrigating solution, or an appropriate wound-cleaning agent). Antiseptic solutions generally aren’t recommended for wound irrigation or dressings because they’re toxic to fibroblasts and other wound-repairing cells. If you must use such a solution, make sure it’s well diluted.
To ensure gentle cleaning or irrigation, pour solution over the wound bed or gently flush the wound with solution (using a 60-mL catheter-tip syringe) until the drainage clears. Know that pressurized irrigation techniques and whirlpool therapy aren’t recommended for wound cleaning because they disturb cell proliferation in the wound bed.
Debriding the wound. Debridement removes slough and necrotic tissue. Nonselective debridement techniques remove any type of tissue within the wound bed, whereas selective methods remove only necrotic tissue. (See Wound debridement techniques by clicking the PDF icon below.)
Maintaining moisture balance
To maintain moisture balance in the wound bed, you must manage exudate and keep the wound bed moist. The proper dressing (which may stay in place for days or longer) supports moist wound healing and exudate management. To minimize fluid pooling, a drain may be inserted into the wound. Negative-pressure wound therapy also may aid removal of excess exudate.
Choosing an appropriate dressing
The wound dressing plays a major role in maintaining moisture balance. Dressing selection is challenging because of the large number and variety of dressings available. Each product has specific actions, benefits, and drawbacks, so determining which dressing best suits the patient’s needs is a multifaceted process.
Dressing choice depends on such factors as wound type and appearance, exudate, presence or absence of pain, and required dressing change frequency. (See Dressings Options by clicking the PDF icon above.)
In a traditional dressing, gauze is applied in layers. The initial (contact) layer in the wound bed absorbs drainage and wicks it to the next layer; most often, this layer consists of woven cotton gauze or synthetic gauze. Remove the gauze gently, because it may be stuck to the wound or incision (especially if the gauze is cotton). For easier removal, moisten the dressing with normal saline solution to loosen it.
With a traditional dressing, the cover layer or secondary dressing is an abdominal pad with a “no-strike-through” layer next to the outside of the dressing. Be aware that wet-to-dry dressings are highly discouraged for their nonselective debriding effect and inability to provide a moist wound bed.
Reassess the patient’s wound at least weekly (after preparing the wound bed and dressing the wound) to determine healing progress. Keep in mind that wound-care management is a collaborative effort. Once you’ve assessed the patient, discuss your findings and subsequent wound management with other members of the team.
Wound care wisdom
Getting wiser about wound care will help your patients achieve good outcomes. Poor wound healing can be frustrating to patients, family members, and healthcare providers alike. Chronic wounds may necessitate lifestyle changes and lead to severe physical consequences ranging from infection to loss of function and even death. By performing careful assessment, tailoring patients’ wound care to wound etiology, and using evidence-based protocols to manage wounds, you can promote speedier wound healing, help lower morbidity, and improve quality of life.
Selected references
Bryant RA, Nix DP. Acute and Chronic Wounds: Current Management Concepts. 4th ed. St. Louis, MO: Mosby; 2011.
Gardener SE, Frantz R, Hillis SL, Park H, Scherubel M. Diagnostic validity of semiquantitative swab cultures. Wounds. 2007;(19)2:31-38.
Krasner DL, Rodeheaver GT, Sibbald RG. Chronic Wound Care: A Clinical Source Book for Healthcare Professionals. 4th ed. Wayne, PA: HMP Communications; 2007.
Langemo DK, Brown G. Skin fails too: acute, chronic, and end-stage skin failure. Adv Skin Wound Care. 2006;19(4):206-211.
Langemo DK, Anderson J, Hanson D, Hunter S, Thompson P. Measuring wound length, width, and area: which technique? Adv Skin Wound Care. 2008;21:42-45.
Milne C, Armand OC, Lassie M. A comparison of collagenase to hydrogel dressings in wound debridement. Wounds. 2010:22(11):270-274.
National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisory Panel. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Washington, DC: National Pressure Ulcer Advisory Panel; 2009.
Ovington LG. Hanging wet-to-dry dressings out to dry. Adv Skin Wound Care. 2002;15(2):79-86.
Sibbald RG, Coutts P, Woo KY. Reduction of bacterial burden and pain in chronic wounds using a new polyhexamethylene biguanide antimicrobial foam dressing—clinical trial results. Adv Skin Wound Care. 2011;24(2):78-84.
Solway DR, Consalter M, Levinson DJ. Microbial cellulose wound dressing in the treatment of skin tears in the frail elderly. Wounds. 2010:22(1):17-19.
Wound Ostomy and Continence Nurses Society. Guideline for Prevention and Management of Pressure Ulcers. Mt. Laurel, NJ: Author; 2010
Patricia A. Slachta is a Clinical Nurse Specialist at The Queens Medical Center in Honolulu, Hawaii and an adjunct nursing instructor at the Technical College of the Lowcountry in Beaufort, South Carolina.
Read MoreWound Healing Improves With New Bioactive Peptide Combo
By combining bioactive peptides, researchers have successfully stimulated wound healing in an in vitro and in vivo study. The studies, published in PLoS ONE, show that the combination of two peptides stimulates growth of blood vessels and promotes tissue re-growth of tissue. Further research into these peptides could potentially lead to new therapies for chronic and acute wounds.
The researchers evaluated a newly-created peptide, UN3, in pre-clinical models with the goal of simulating impaired wound healing as in patients suffering from peripheral vascular diseases or uncontrolled diabetes. They discovered that the peptide increased the development of blood vessel walls by 50%, with an 250% increase in blood vessel growth, and a 300% increase in cell migration in response to the injury. (more…)
Read MoreWhy do older people heal more slowly?
By Matthew Steinhauser, University of Pittsburgh
I recently visited an 83-year-old patient in the hospital after EMTs rushed her to the ER with an infected leg wound. Her ordeal started inconspicuously when she bumped into the sharp edge of a table and developed a small cut. The patient’s wound didn’t close, but she ignored it until she woke up in pain one morning two weeks after first injuring her leg. Her daughter called 911 after noticing angry, red skin discoloration and pus – both signs of an infection. Our medical team treated her with IV antibiotics and cleared up the infection, but the wound did not fully close until at least a month later, well after she was discharged from the hospital.
How different the story is when children get a cut. They may scream initially, but within days, the scab falls off, revealing new skin. Why was healing so delayed in my 83-year-old patient compared to a healthy child?
The answer is age. Decades of life slow down healing for most tissues, and wounds in skin can offer a window into why this slowdown occurs.
Three stages of wound healing
I am physician who studies how aging predisposes patients to diseases like diabetes and whether behavioral changes such as intermittent fasting may slow down aging. In order to understand why the skin wound in my older patient healed so slowly, it is important to first understand how wounds heal under the ideal conditions of youth.
The wound healing process is classically categorized into three stages.
Right after a wound occurs, the inflammatory response begins.
Jpbarrass via Wikimedia Commons
The first stage is inflammation, essentially the body’s attempt to clean the wound. During the inflammatory phase, immune cells called phagocytes move into the wound, kill any contaminating bacteria, and ingest and dispose of dead cells and debris.
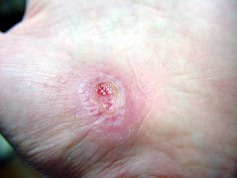
Jpbarrass via Wikimedia Commons
Inflammation sets the stage for the regenerative phase, where several processes work in concert to regrow damaged skin. Replacement skin cells are born when cells at the edge of the wound divide, while fibroblast cells lay down a supportive scaffolding called the extracellular matrix. This holds the new cells together. Any damaged supporting structures of the skin, such as the blood vessels that supply critical oxygen and nutrients, also need to regrow. The second stage effectively closes the wound and restores a protective barrier against bacteria.
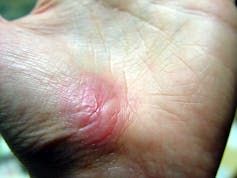
Jpbarrass via Wikimedia Commons
The regenerative phase is a relatively quick, but tenuous fix – new skin is fragile. The final remodeling phase plays out over a couple of years as the new skin is progressively strengthened by several parallel processes. The extracellular matrix, which was initially laid down in a haphazard fashion, is broken down and replaced in a more durable way. Any residual cells from prior phases that are no longer needed – such as immune cells or fibroblasts – become inactive or die. In addition to strengthening the new skin, these collective actions also account for the tendency of scars to visibly fade with time.
Products
Diseases disrupt the healing process
One major way aging can derail the orderly and efficient progression through the stages of healing is through the health problems that stem from diseases of old age.
Diabetes is one example of a disease that is strongly associated with older age. One of the many ways that diabetes negatively affects healing is by causing blood vessels to narrow. As a consequence of inadequate circulation, crucial nutrients and oxygen do not reach the wound in sufficient quantities to fuel the second regenerative phase.
Diabetes is just one of many age-related diseases that disrupts normal processes in the body such as wound healing.
Cells age too
Aside from the negative impacts of age-associated diseases, cells themselves age. In an extreme sign of aging called cellular senescence, cells permanently lose the ability to divide. Senescent cells accumulate in skin and many other organs as people age and cause a host of problems.
When cells divide more slowly – or when they stop dividing altogether due to senescence – skin becomes thinner. The replacement of fat cells, which form a cushioning layer under the skin, also declines with age. The skin of older patients is therefore more prone to injury in the first place.
Once an older person’s skin is injured, the skin has a harder time healing properly as well. Aging and senescent immune cells cannot defend against bacteria, and the risk of serious skin infection rises. Then in the regenerative stage, slow rates of cell division translate into slow skin regrowth. My patient exhibited all of these negative effects of age – her thin, almost translucent skin ruptured from a minor bump, became infected and took nearly two months to fully regrow.
But senescent cells are more than just dysfunctional bystanders. For reasons that are not yet fully understood, senescent cells release toxic byproducts that damage surrounding tissue and drive inflammation – even when there’s no bacterial threat present. Some of these byproducts can even accelerate senescence in neighboring cells. This suggests that intrinsic aging of cells is in essence contagious and senescent cells actively fuel an uncontrolled cycle of inflammation and tissue damage that further impedes successful regeneration and healing.
A whole body problem
As the most outwardly visible tissue of the body, the skin provides a window into why people heal more slowly with age, but all tissues can be injured and are susceptible to the effects of aging. Injuries may be small, repetitive and build up over time – like the effect of smoking on the lungs. Or they may be discrete and dramatic – such as the death of heart cells with a heart attack. Different tissues may heal in different ways. Yet all tissues share a sensitivity to the repercussions of an aging immune system and a decline in the ability to regrow dead or damaged cells.
Understanding why healing slows down with age is important, but my patient asked a very practical question that physicians often face in one form or another: “Doctor, what can you do for me?”
Unfortunately, current treatment of wounds is fairly old-fashioned and often ineffective. Some of the options available include wound dressing changes, antibiotics when the wound is infected or treatment in a high oxygen chamber when circulation is bad due to diabetes.
There is hope, though, that medicine can do better and that progress in understanding the aging process will lead to new therapies. Neutralizing senescent cells in mice, for example, improves a variety of age-associated diseases. While it is way too early to say that researchers have discovered the fountain of youth, I am optimistic for a future when physicians will bend the aging curve and make skin and other organs heal faster and better.
Matthew Steinhauser, Associate Professor of Medicine, University of Pittsburgh
This article is republished from The Conversation under a Creative Commons license. Read the original article.
A young Black scientist discovered a pivotal leprosy treatment in the 1920s − but an older colleague took the credit
By Mark M. Lambert, Des Moines University
Hansen’s disease, also called leprosy, is treatable today – and that’s partly thanks to a curious tree and the work of a pioneering young scientist in the 1920s. Centuries prior to her discovery, sufferers had no remedy for leprosy’s debilitating symptoms or its social stigma.
This young scientist, Alice Ball, laid fundamental groundwork for the first effective leprosy treatment globally. But her legacy still prompts conversations about the marginalization of women and people of color in science today.
As a bioethicist and historian of medicine, I’ve studied Ball’s contributions to medicine, and I’m pleased to see her receive increasing recognition for her work, especially on a disease that remains stigmatized.
Who was Alice Ball?
Alice Augusta Ball, born in Seattle, Washington, in 1892, became the first woman and first African American to earn a master’s degree in science from the College of Hawaii in 1915, after completing her studies in pharmaceutical chemistry the year prior.
After she finished her master’s degree, the college hired her as a research chemist and instructor, and she became the first African American with that title in the chemistry department.
Impressed by her master’s thesis on the chemistry of the kava plant, Dr. Harry Hollmann with the Leprosy Investigation Station of the U.S. Public Health Service in Hawaii recruited Ball. At the time, leprosy was a major public health issue in Hawaii.
Doctors now understand that leprosy, also called Hansen’s disease, is minimally contagious. But in 1865, the fear and stigma associated with leprosy led authorities in Hawaii to implement a mandatory segregation policy, which ultimately isolated those with the disease on a remote peninsula on the island of Molokai. In 1910, over 600 leprosy sufferers were living in Molokai.
This policy overwhelmingly affected Native Hawaiians, who accounted for over 90% of all those exiled to Molokai.
The significance of chaulmoogra oil
Doctors had attempted to use nearly every remedy imaginable to treat leprosy, even experimenting with dangerous substances such as arsenic and strychnine. But the lone consistently effective treatment was chaulmoogra oil.
Chaulmoogra oil is derived from the seeds of the chaulmoogra tree. Health practitioners in India and Burma had been using this oil for centuries as a treatment for various skin diseases. But there were limitations with the treatment, and it had only marginal effects on leprosy.
The oil is very thick and sticky, which makes it hard to rub into the skin. The drug is also notoriously bitter, and patients who ingested it would often start vomiting. Some physicians experimented with injections of the oil, but this produced painful pustules.

The Ball Method
If researchers could harness chaulmoogra’s curative potential without the nasty side effects, the tree’s seeds could revolutionize leprosy treatment. So, Hollmann turned to Ball. In a 1922 article, Hollmann documents how the 23-year-old Ball discovered how to chemically adapt chaulmoogra into an injection that had none of the side effects.
The Ball Method, as Hollmann called her discovery, transformed chaulmoogra oil into the most effective treatment for leprosy until the introduction of sulfones in the late 1940s.
In 1920, the Ball Method successfully treated 78 patients in Honolulu. A year later, it treated 94 more, with the Public Health Service noting that the morale of all the patients drastically improved. For the first time, there was hope for a cure.
Tragically, Ball did not have the opportunity to revel in this achievement, as she passed away within a year at only 24, likely from exposure to chlorine gas in the lab.
Ball’s legacy, lost and found
Ball’s death meant she didn’t have the opportunity to publish her research. Arthur Dean, chair of the College of Hawaii’s chemistry department, took over the project.
Dean mass-produced the treatment and published a series of articles on chaulmoogra oil. He renamed Ball’s method the “Dean Method,” and he never credited Ball for her work.
Ball’s other colleagues did attempt to protect Ball’s legacy. A 1920 article in the Journal of the American Medical Association praises the Ball Method, while Hollmann clearly credits Ball in his own 1922 article.
Ball is described at length in a 1922 article in volume 15, issue 5, of Current History, an academic publication on international affairs. That feature is excerpted in a June 1941 issue of Carter G. Woodson’s “Negro History Bulletin,” referring to Ball’s achievement and untimely death.
Joseph Dutton, a well-regarded religious volunteer at the leprosy settlements on Molokai, further referenced Ball’s work in a 1932 memoir broadly published for a popular audience.
Historians such as Paul Wermager later prompted a modern reckoning with Ball’s poor treatment by Dean and others, ensuring that Ball received proper credit for her work. Following Wermager’s and others’ work, the University of Hawaii honored Ball in 2000 with a bronze plaque, affixed to the last remaining chaulmoogra tree on campus.
In 2019, the London School of Hygiene and Tropical Medicine added Ball’s name to the outside of its building. Ball’s story was even featured in a 2020 short film, “The Ball Method.”
The Ball Method represents both a scientific achievement and a history of marginalization. A young woman of color pioneered a medical treatment for a highly stigmatizing disease that disproportionately affected an already disenfranchised Indigenous population.
In 2022, then-Gov. David Ige declared Feb. 28 Alice Augusta Ball Day in Hawaii. It was only fitting that the ceremony took place on the Mānoa campus in the shade of the chaulmoogra tree.
Mark M. Lambert, Assistant Professor of Behavioral Medicine, Medical Humanities, and Bioethics, Des Moines University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Read More