By Leanne Richbourg, MSN, RN, APRN-BC, CWON-AP, CCCN, GCNS-BC
Restorative proctocolectomy with ileal pouch anal anastomosis (IPAA) is the gold standard for surgical treatment of ulcerative colitis (UC) or familial adenomatous polyposis (FAP). It’s also done to treat colon and rectal cancers, such as those caused by Lynch syndrome (LS). IPAA allows the patient to maintain fecal continence and evacuate stool from the anus after colon and rectum removal. A temporary ileostomy may be part of the overall process, but there’s no need for a permanent stoma. (See Understanding ulcerative colitis, FAP, and Lynch syndrome.)
Contraindications for IPAA include:
- Crohn’s disease, which can recur at any point along the GI tract
- incompetent anal sphincter tone (most common in older adults)
- diseases of the distal rectum or anal canal.
Preoperative education
You can help improve your patient’s quality of life and health status by providing education about IPAA. When preparing the patient for surgery, explain the procedure and discuss how it will change GI tract anatomy. Because the patient is likely to have a stoma, describe what the stoma will look like, how to care for it, how to use pouches to contain stoma output, what lifestyle adjustments to expect, and psychological preparation.
Explain that by the 12th postoperative month, the patient’s physical and psychological health, independence level, and general overall quality of life is likely to improve significantly over preoperative levels. By 3 years, quality of life scores most likely will match those of the healthy population in terms of physical health, independence, spirituality, and environment. Psychological health and social relationships scores also typically improve, although not quite to the extent as the healthy population.
Surgical technique
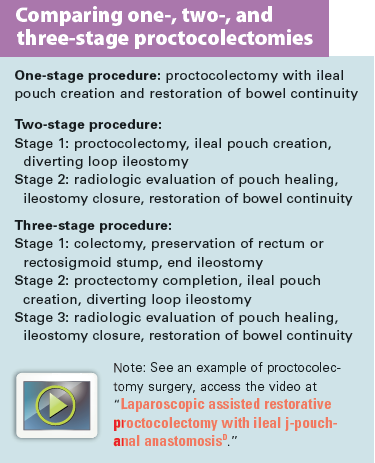
Proctocolectomy with IPAA can be done as a one-, two- or three-stage procedure. (See Comparing types of proctocolectomies).
Taking care to preserve the pelvic nerves, the surgeon creates a reservoir from 30 cm to 40 cm of distal ileum and connects it to the anal canal at or just above the dentate line (where columnar epithelium transitions to squamous epithelium). The most common pouch configuration is the two-limbed pouch, called the J-pouch.
Although two-stage surgery with a diverting loop ileostomy is the most common, a three-stage procedure is optimal for patients who are markedly debilitated by disease due to severe exacerbations, nutritional compromise, and high-dose steroid therapy. For those with indeterminate colitis and suspected Crohn’s disease, step one of the three-stage procedure allows for further testing before ileal pouch creation. The one-stage procedure without a diverting ileostomy is linked to increased risk of pouch leakage, pelvic infection, and subsequent pouch failure.
Proctocolectomy with IPAA may be performed through an open abdominal incision or a laparoscopic approach. Laparoscopic surgery takes an average of 80 minutes longer and requires more I.V. fluids. However, it’s associated with shorter hospital stays, shorter time to ostomy closure, shorter operating-room times, shorter stays for ostomy reversal surgery, less adhesion formation, and lower infertility rates. The two methods don’t differ in blood loss, need for postoperative opioids, return of bowel function, or hospital readmission rates. An open abdominal approach usually is done in patients with fulminant colitis or acute colitis complicated by colonic perforation or toxic megacolon.
Complications
During the first postoperative month, symptomatic portal-vein thrombosis occurs in up to 6% of patients; asymptomatic clots may arise in up to 40%. The cause is unknown but may relate to traction on the superior mesenteric vein when the small bowel moves down into the pelvis in patients with systemic inflammation from ulcerative colitis. Signs and symptoms may mimic those of an acute abdomen, including nausea, vomiting, fever, abdominal distention, and pain. Computed tomography is used to identify thrombi. Treatment involves 3 to 6 months of anticoagulation.
Postoperative management after ileostomy creation
Initially, your main role is to provide education about ileostomy self-care. Teach your patient how to apply, empty, and replace the ostomy pouch. (See Providing education to the new ostomate.)
After surgery, high stool output from the diverting ileostomy is a common problem. Although definitions of high output differ somewhat, I tell patients that high output means more than 1,200 mL in 24 hours. This condition is most common within the first 2 to 3 postoperative weeks and usually resolves.
Readmission for dehydration typically occurs during the second postop week. To help prevent dehydration, instruct patients to drink eight to ten 8-oz glasses of fluid daily, preferably avoiding fruit juice, soft drinks containing sugar, caffeinated drinks, and alcohol.
Measuring stool output
Advise the patient to keep track of how much stool he or she is passing in 24 hours. I usually advise patients to empty the pouch when it’s one-third to one-half full, four to six times daily. This equates to about 1,200 mL of output. If the pouch requires more frequent emptying, the patient needs to quantify the output further.
I’ve found some discharged patients don’t comply with measuring stool output using a urinal or “potty hat.” So I give patients a photograph of three pouches containing colored water in quantities of 100 mL, 200 mL, and 300 mL. This helps them visually judge how much output is in the pouch. Antidiarrheal medications, such as loperamide, can be titrated to keep output within the normal range.
About 6 weeks after surgery, instruct the patient to practice pelvic floor muscle exercises three times daily, if the surgeon approves. This strengthens the muscles needed for fecal continence once the stoma is closed.
Postoperative management after ileostomy closure
After ileostomy closure, patients with an ileoanal pouch must begin training their new reservoir—a process that can take up to a year. Initially, they may have up to 20 small bowel movements daily and may need to get out of bed multiple times at night to pass stool. Eventually, this decreases to six bowel movements in 24 hours, including overnight. At 1 year postop, about 95% of patients who’ve had ileoanal pouch surgery report being very satisfied with their decision. For the remaining 5%, long-term functional results are poor.
To slowly increase pouch holding capacity, teach patients not to respond to every urge to move the bowels. Initially, advise them to wait 5 minutes after sitting on the toilet before responding. When that’s accomplished, tell them to increase the wait time by another 5 minutes, and then 10 minutes, and to continue increasing it in this manner. Also advise them to begin to move further and further away from the toilet during wait time. The goal is to gain confidence in the ability to withhold stool and avoid accidents.
Complications of ileal pouch surgery include pouchitis; pouch-anal anastomotic strictures; small-bowel obstruction; and intra-abdominal, peri-pouch, and anastomotic cuff abscesses. (See Understanding potential complications.)
Perianal skin care
Stool leakage is common at first, along with trouble differentiating between flatus and stool. Teach patients how to perform meticulous perianal skin care. Tell them to wash the area thoroughly at least once daily using a pH-balanced skin cleanser and warm water. To clean up after each bowel movement, advise them to use alcohol-free moist wipes, which are less abrasive than toilet paper.
Encourage patients to protect perianal skin by applying an ointment containing petrolatum, dimethicone, or zinc oxide after each bowel movement. Alternatively, they may use an alcohol-free liquid skin-barrier film wipe once daily. Aloe vera gel is an effective treatment for skin irritation, providing antimicrobial action, reducing pain, and shortening healing times. Depending on leakage amounts, advise patients to protect underclothes with a panty liner or incontinence-containment product. Encourage them to continue regular pelvic floor muscle exercises, as strong muscles are crucial for preventing leakage.
Dietary guidelines
Advise patients to eat a low-fiber diet for about 4 weeks after ostomy closure, until bowel swelling resolves. Then instruct them to start increasing fiber intake until stools become firmer. Inform them that foods that can contribute to anal irritation include spicy foods and foods high in insoluble fiber, such as stringy fruits and vegetables (oranges, coleslaw, celery, corn, nuts, popcorn, coconut, and Chinese vegetables). Teach patients that stool with a thicker consistency is less likely to leak. (See Foods that thicken stool.)
When stool is thin and frequent, urge patients to eat potassium-rich foods, such as meat, banana, apricots, tomatoes, milk, and potatoes. Tell them they may need to add salt to their food to replace potassium and sodium lost through diarrhea and other fluid losses.
Explain that foods and beverages high in sugar or caffeine can worsen diarrhea. Tell patients to limit fruit juice, caffeinated tea and coffee, honey, candy, sugary and caffeinated soft drinks, chocolate, and baked goods high in sugar.
Teach patients to add 1 tsp of soluble fiber, such as psyllium husks (Metamucil), to 1 cup of fluid one or more times per day, titrated to maintain a more solid stool consistency. To prevent dehydration, encourage them to continue to drink eight to ten 8-oz cups of fluid daily.
For patients who continue to have large quantities of thin stool, recommend an antidiarrheal medication, such as loperamide (Imodium), if the surgeon permits. Instruct them to start with one 2-mg dose 30 minutes before breakfast, lunch, and dinner and another dose at bedtime. If this isn’t effective, tell them they may double the dose, not to exceed 16 mg per 24 hours. Know that some patients will have to stay on this medication for a long time.
You can improve patient outcomes
As the ostomy specialist clinician, your role is to assist patients along the continuum from illness to heath. Providing thorough patient education throughout this process is crucial to helping them achieve their ultimate goal of wellness.
Selected references
American Society of Clinical Oncology. Familial Adenomatous Polyposis. September 2015. cancer
.net/cancer-types/familial-adenomatous-polyposis
Bartels SA, D’Hoore A, Cuesta MA, et al. Significantly increased pregnancy rates after laparoscopic restorative proctocolectomy: a cross-sectional study. Ann Surg. 2012;256(6):1045-8.
Fajardo AD, Dharmarajan S, George V, et al. Laparoscopic versus open 2-stage ileal pouch: laparoscopic approach allows for faster restoration of intestinal continuity. J Am Coll Surg. 2010:211(3);377-83.
Gionchetti P, Calafiore A, Riso D, et al. The role of antibiotics and probiotics in pouchitis. Ann Gastroenterol. 2012;25(2):100-5.
Heikens JT, de Vries, J, Goos, MR, et al. Quality of life and health status before and after ileal pouch anal anastomosis for ulcerative colitis. Br J Surg. 2012;99(2);263-9.
Heikens JT, de Vries J, Goos MR et al. Evaluation of long-term function, complications, quality of life and health status after restorative proctocolectomy with ileo neo rectal and with ileal pouch anal anastomosis for ulcerative colitis. Colorectal Dis. 2013; 15(6);e323-9.
Hiranyakas A, Rather A, da Silva G, et al. Loop ileostomy closure after laparoscopic versus open surgery: is there a difference? Surg Endosc. 2013; 27(1):90-4.
Holubar SD, Cima RR, Sandborn WJ, et al. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev. 2010;6;CD001176.
Hull TL, Joyce MR, Geisler DP, et al. Adhesions after laparoscopic and open ileal pouch-anal anastomosis surgery for ulcerative colitis. Brit J Surg. 2012;99(2);270-5.
Larsen S, Bendtzen K, Nielsen OH. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann Med. 2010;42(2):97-114.
National Cancer Institute. Genetics of Colorectal Cancer–for health professionals. Updated July 6, 2015. cancer.gov/types/colorectal/hp/colorectal-genetics-pdq/#link/_89
Psillos AI, Catanzaro J. Ileal pouch anal anastomosis: an overview of surgery, recovery, and achieving postsurgical continence. Ostomy Wound Manage. 011;57(12);22-8.
Sagar PM, Pemberton JH. Intraoperative, postoperative and reoperative problems with ileoanal pouches. Br J Surg. 2012;99(4):454-68.
Stocchi L. Laparoscopic surgery for ulcerative colitis. Clin Colon Rectal Surg. 2010;23(4):248-58.
Wound, Ostomy, and Continence Nurses Society (WOCN). Management of the Patient With a Fecal Ostomy: Best Practice Guideline for Clinicians. Mount Laurel, NJ: WOCN; 2010.
Leanne Richbourg is a wound and ostomy clinical nurse specialist at Duke University Hospital
in Durham, North Carolina.
Online Resources
A. https://www.youtube.com/watch?v=zDXS0QBGoKY
B. https://www.youtube.com/watch?v=F6pqpLRGneE
C. https://www.youtube.com/watch?v=JMApMBY0CfQ
D. https://www.youtube.com/watch?v=rEYzh8VKqEE
DISCLAIMER: All clinical recommendations are intended to assist with determining the appropriate wound therapy for the patient. Responsibility for final decisions and actions related to care of specific patients shall remain the obligation of the institution, its staff, and the patients’ attending physicians. Nothing in this information shall be deemed to constitute the providing of medical care or the diagnosis of any medical condition. Individuals should contact their healthcare providers for medical-related information.