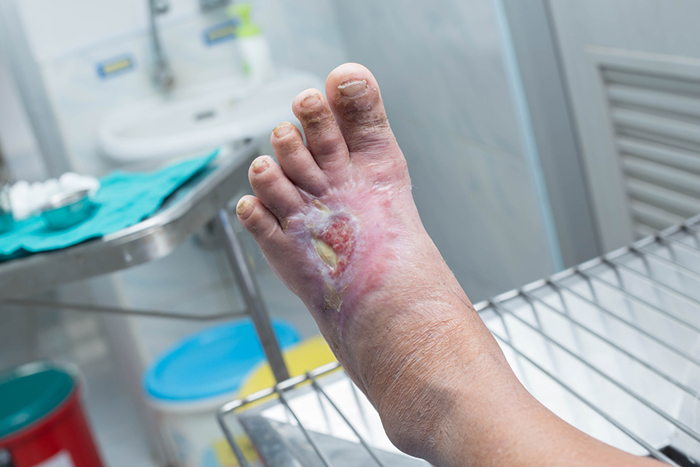
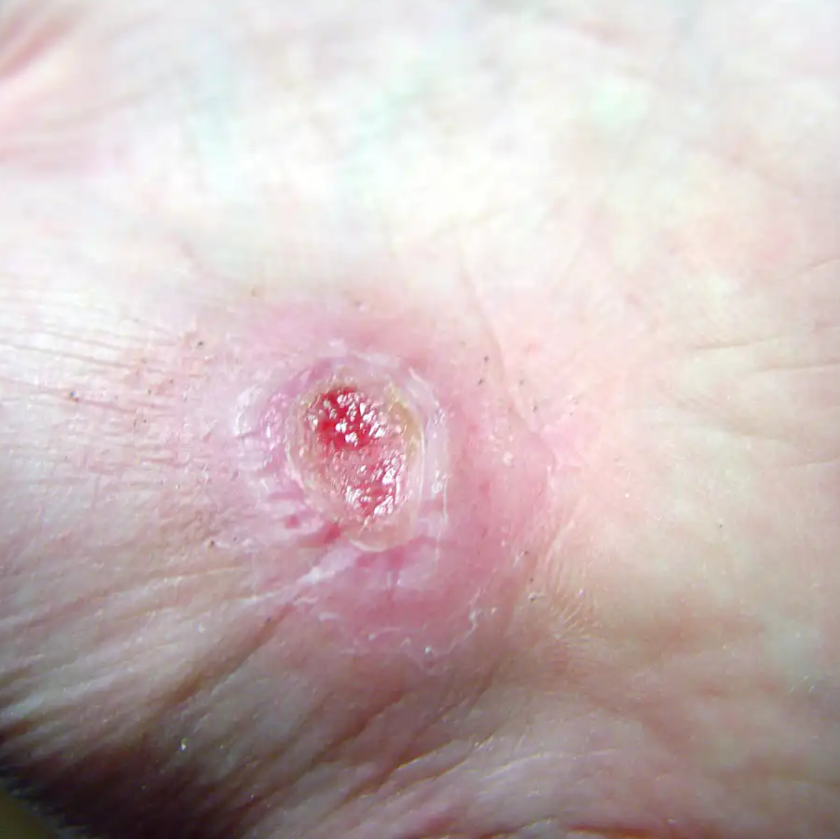
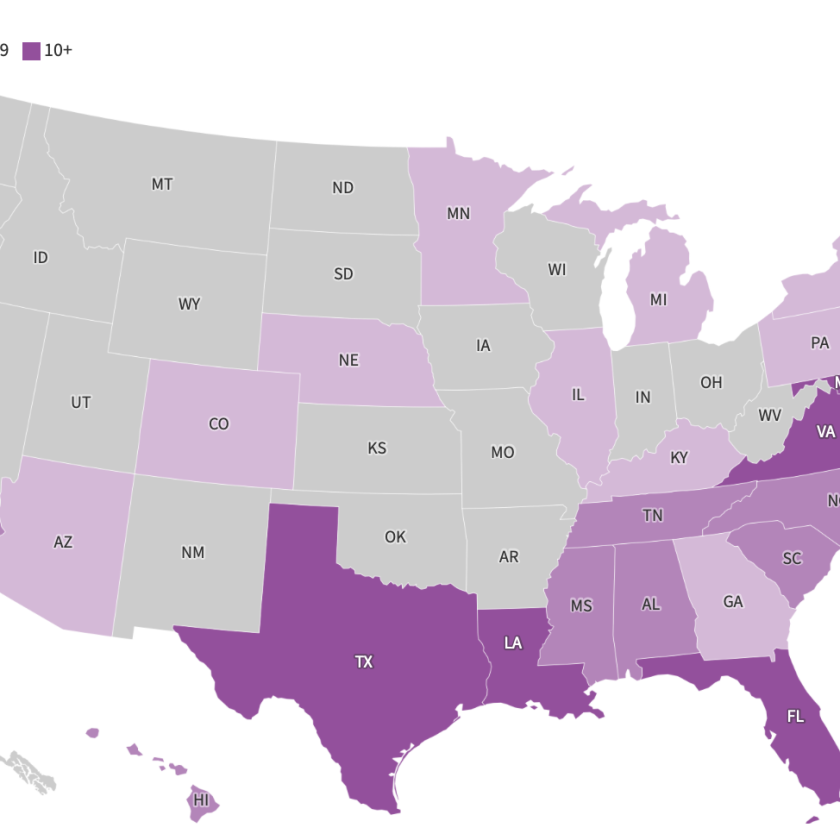
On December 28, 2017, the FDA gave approval for the Dermapace System, a shock wave device intended to be used in the treatment of chronic, full-thickness diabetic foot ulcers. The device uses pulses of energy, similar to sound waves, to mechanically stimulate the wound. Read more.
FDA approves shock wave device for treatment of diabetic foot ulcers