By: Donna Sardina, RN, MHA, WCC, CWCMS, DWC, OMS
A medical device–related pressure ulcer (MDRPU) is defined as a localized injury to the skin or underlying tissue resulting from sustained pressure caused by a medical device, such as a brace; splint; cast; respiratory mask or tubing; tracheostomy tube, collar, or strap; feeding tube; or a negative-pressure wound therapy device. The golden rule of pressure ulcer treatment is to identify the cause of pressure and remove it. Unfortunately, many of the medical devices are needed to sustain the patient’s life, so they can’t be removed.
But does that mean MDRPUs aren’t avoidable? Yes—and no. Some aren’t avoidable, but not as many as you might think. Many result not from the device itself but from poor device positioning or securement. Some result from simple failure to check under the tubing or device. These causes are avoidable. Preventive practices include frequently evaluating device positioning and securement. Also, if possible, loosen the device at least once per shift to check for skin problems.
The National Pressure Ulcer Advisory Panel has created four “Best Practices for Prevention of Medical Device–Related Pressure Ulcers” posters, which can be downloaded for free. Besides a general poster, you’ll find posters for the specialties of critical care, pediatrics, and long-term care. Each poster features photos of MDRPU-related injuries and prevention strategies such as:
- Choose the correctly sized medical device for the individual.
- Cushion and protect the skin with dressings in high-risk areas.
- Remove or move the device daily to assess skin.
- Avoid placing the device over the site of a previous or existing pressure ulcer.
- Educate staff on the correct use of devices and prevention of skin breakdown.
- Be aware of edema under the device and the potential for skin breakdown.
- Confirm that the device isn’t placed directly under a patient who’s bedridden or immobile.
Of course, even when caregivers focus on prevention, mistakes can happen. Unfortunately, mistakes are a part of life. But that doesn’t mean we can’t learn from our mistakes to better protect our patients. When a mistake occurs, determine what happened, correct it, and take steps to prevent it from happening again. That’s our job as clinicians and as patient advocates.
Donna Sardina, RN, MHA, WCC, CWCMS, DWC, OMS
Editor-in-Chief
Wound Care Advisor
Cofounder, Wound Care Education Institute
Plainfield, Illinois
Selected references
Black JM, Cuddigan JE, Walko MA, Didier LA, Lander
MJ, Kelpe MR. Medical device related pressure
ulcers in hospitalized patients. Int Wound J.
2010;7(5):358–65.
Fletcher J. Device related pressure ulcers made easy.
Wounds UK. 2012;8(2). www.woundsinternational
.com/pdf/content_10472.pdf. Accessed June 23, 2014.
DISCLAIMER: All clinical recommendations are intended to assist with determining the appropriate wound therapy for the patient. Responsibility for final decisions and actions related to care of specific patients shall remain the obligation of the institution, its staff, and the patients’ attending physicians. Nothing in this information shall be deemed to constitute the providing of medical care or the diagnosis of any medical condition. Individuals should contact their healthcare providers for medical-related information.

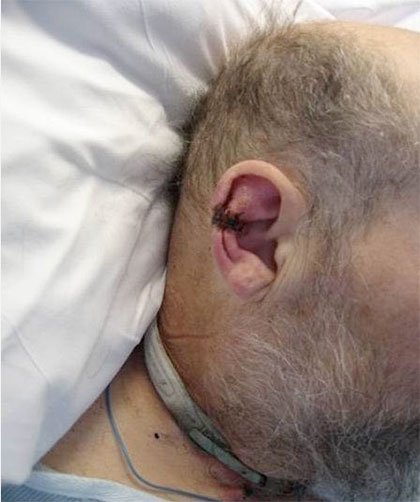
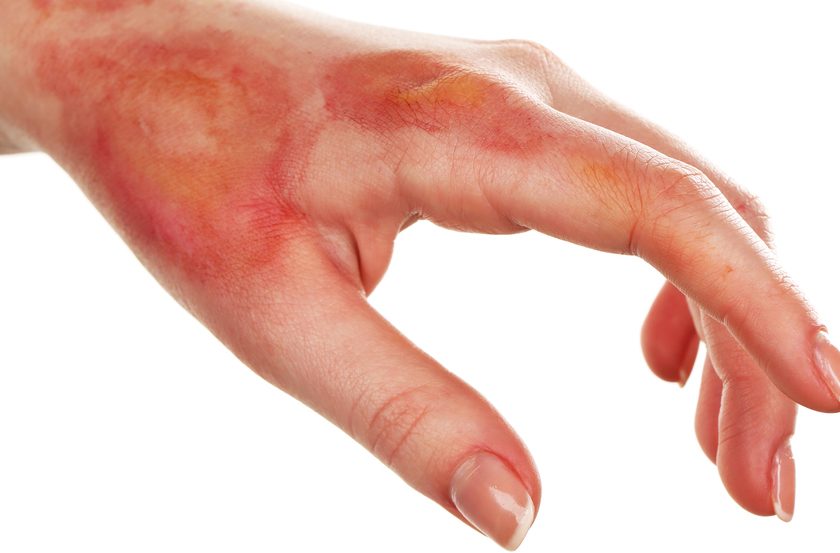
In the pediatric world here at Cincinnati Childrens Hospital Medical Center we have been dealing with and preventing pressure ulcers from devices for years and have had great success with prevention.
One of the greatest challenges was with BIPAP. It is only recently that we have been able to get some masks that are made for pediatric pts but we still use many adult masks that do not fit properly. As a result we see PU’s. We developed a protocol which has tremendously decreased our numbers. This protocol consist of the following:
1. Wash face and mask once a day with
soap and water
2. Remove mask Q4H for mouth care and
give pressure relief
3. DO NOT PLACE ANYTHING UNDER THE
MASK UNTIL REDNESS BEGINS. IF
REDNESS DEVELOPS USE HYDROGEL
(SUCH AS CARADRES) THEN MEPILEX
LITE ON THE RED AREA ONLY.
REPLACE Q4H DUE TO DRYING OUT.
(this has actually shown to reverse the redness that develops and prevents PU development)
4. Alternate between two different mask (full
face vs shield) Q4H
We have also developed other protocols for trachs c collars pulse ox. if interested
Mary Ann,
I work in an ICU and am on the skin care committee. We are currently doing a quality improvement project to help improve our HAPU numbers. I am very interested in your other protocols that you use for other devices. Also, is it okay if I share your protocols with my unit. I am doing a power point presentation on prevention of MDRPUs and fined your face mask protocol to be very good.