A glass-based wound care product that emerged from research by a doctoral student at Missouri University of Science and Technology has been approved by the U.S. Food and Drug Administration for human use and is now available on the commercial market.
Steve Jung laid the groundwork for the Mirragen Advanced Wound Matrix while earning a master’s degree in ceramic engineering and a Ph.D. in materials science and engineering at Missouri S&T. Jung is now chief technology officer at Mo-Sci Corp., a Rolla specialty glass manufacturer that continued the product’s development in collaboration with ETS Wound Care, also of Rolla.
“The recent FDA approval is a significant milestone,” says Chad Lewis, president and CEO of ETS Wound Care, a subsidiary of Engineered Tissue Solutions. “We’re pioneering an entirely new therapeutic option for wound care.”
ETS Wound Care initially plans a controlled domestic market release of Mirragen while making the product available to the broader domestic market in 2018. The company is exhibiting its new product at the spring Symposium on Advanced Wound Care on April 6-8 in San Diego.
Jung’s doctoral adviser at S&T was Dr. Delbert Day, a groundbreaking glass engineer who co-founded Mo-Sci more than three decades ago as a spinoff company to manufacture radioactive glass spheres that he and his students had developed to treat patients with inoperable liver cancer. Day, a member of the National Academy of Engineering and a forthcoming inductee into the National Academy of Inventors, is now a Curators’ Distinguished Professor emeritus of materials science and engineering at S&T.
“As a student, it’s hard to understand the potential impacts of your work,” says Jung, who also received a Missouri S&T bachelor’s degree. “So it’s very exciting to now see this research commercialized to do exactly what was intended – to help people.”

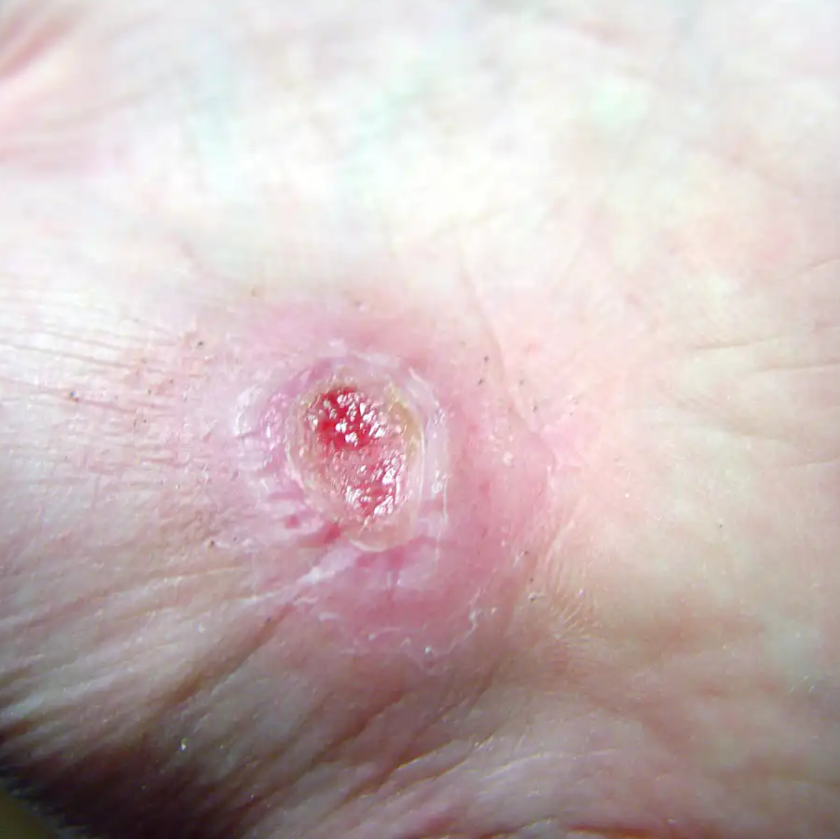
The Mirragen Advanced Wound Matrix is a wound dressing solely composed of microscopic glass fibers and particles that are absorbed by the body. Both flexible and moldable, the wound dressing can be easily customized, while its fiber structure allows Mirragen to absorb fluid from the wound site and facilitate healing.
“Anyone who is treating or experiencing discomfort from acute or chronic wounds will immediately recognize its benefits,” says Peggy Earl, a wound care specialist at Phelps County Regional Medical Center in Rolla, where clinical trials of were conducted. “It has the potential to reduce the required number of episodes and duration of wound care treatment, while allowing the body to effectively heal a variety of wounds, both acute and chronic.”
A similar product, Rediheal, also stemming from Jung’s research at S&T, has been successfully used by veterinarians for the past three years to heal major wounds in animals.
Keith Strassner, director of the university’s office of technology transfer and economic development, calls the new wound care product a successful example of the real-world benefits of academic research.
“The Mirragen story perfectly illustrates how federal support of university research can translate into broader economic and social benefits,” he says, noting the early support of Jung’s work by a U.S. Department of Defense grant. “Then, we were able to create a strong partnership with Mo-Sci and transfer the technology to allow the company to make the necessary investments in its commercialization and the regulatory approval process.”