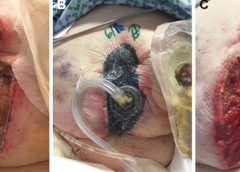
Negative pressure wound therapy (NPWT) uses negative pressure to draw wound edges together, remove edema and infectious material, and promote perfusion and granulation tissue development. The tissue stretch and compression created by negative pressure during NPWT promotes tissue perfusion and granulation tissue development through angiogenesis, cellular proliferation, fibroblast migration, increased production of wound healing proteins, and reduction of wound area. NPWT has been used to improve healing in a variety of wounds, including traumatic injuries, surgical wounds, pressure ulcers, diabetic foot ulcers, and venous stasis ulcers. (more…)
Read MoreTag: negative-pressure wound therapy
Guidelines for safe negative-pressure wound therapy
By Ron Rock MSN, RN, ACNS-BC
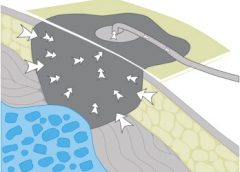
Since its introduction almost 20 years ago, negative-pressure wound therapy (NPWT) has become a leading technology in the care and management of acute, chronic, dehisced, traumatic wounds; pressure ulcers; diabetic ulcers; orthopedic trauma; skin flaps; and grafts. NPWT applies controlled suction to a wound using a suction pump that delivers intermittent, continuous, or variable negative pressure evenly through a wound filler (foam or gauze). Drainage tubing adheres to an occlusive transparent dressing; drainage is removed through the tubing into a collection canister. NWPT increases local vascularity and oxygenation of the wound bed and reduces edema by removing wound fluid, exudate, and bacteria. (more…)
Read More