By Hedy Badolato, RD, CSR, CNSC; Denise Dacey, RD, CDE; Kim Stevens, BSN, RN, CCRN; Jen Fox, BSN, RN, CCRN; Connie Johnson, MSN, RN, WCC, LLE, OMS, DAPWCA; Hatim Youssef, DO, FCCP; and Scott Sinner, MD, FACP
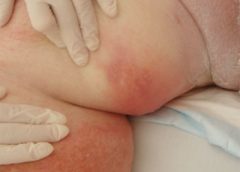
Despite the healthcare team’s best efforts, not all hospitalizations go smoothly. This article describes the case of an obese patient who underwent bariatric surgery. After a 62-day hospital stay, during which a multidisciplinary team collaborated to deliver the best care possible, he died. Although the outcome certainly wasn’t what we wanted, we’d like to share his story to raise awareness of the challenges of caring for bariatric patients. (more…)
Read More