By Mark M. Lambert, Des Moines University
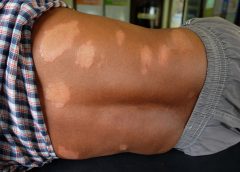
Hansen’s disease, also called leprosy, is treatable today – and that’s partly thanks to a curious tree and the work of a pioneering young scientist in the 1920s. Centuries prior to her discovery, sufferers had no remedy for leprosy’s debilitating symptoms or its social stigma.
This young scientist, Alice Ball, laid fundamental groundwork for the first effective leprosy treatment globally. But her legacy still prompts conversations about the marginalization of women and people of color in science today.
As a bioethicist and historian of medicine, I’ve studied Ball’s contributions to medicine, and I’m pleased to see her receive increasing recognition for her work, especially on a disease that remains stigmatized.
Who was Alice Ball?
Alice Augusta Ball, born in Seattle, Washington, in 1892, became the first woman and first African American to earn a master’s degree in science from the College of Hawaii in 1915, after completing her studies in pharmaceutical chemistry the year prior.
After she finished her master’s degree, the college hired her as a research chemist and instructor, and she became the first African American with that title in the chemistry department.
Impressed by her master’s thesis on the chemistry of the kava plant, Dr. Harry Hollmann with the Leprosy Investigation Station of the U.S. Public Health Service in Hawaii recruited Ball. At the time, leprosy was a major public health issue in Hawaii.
Doctors now understand that leprosy, also called Hansen’s disease, is minimally contagious. But in 1865, the fear and stigma associated with leprosy led authorities in Hawaii to implement a mandatory segregation policy, which ultimately isolated those with the disease on a remote peninsula on the island of Molokai. In 1910, over 600 leprosy sufferers were living in Molokai.
This policy overwhelmingly affected Native Hawaiians, who accounted for over 90% of all those exiled to Molokai.
The significance of chaulmoogra oil
Doctors had attempted to use nearly every remedy imaginable to treat leprosy, even experimenting with dangerous substances such as arsenic and strychnine. But the lone consistently effective treatment was chaulmoogra oil.
Chaulmoogra oil is derived from the seeds of the chaulmoogra tree. Health practitioners in India and Burma had been using this oil for centuries as a treatment for various skin diseases. But there were limitations with the treatment, and it had only marginal effects on leprosy.
The oil is very thick and sticky, which makes it hard to rub into the skin. The drug is also notoriously bitter, and patients who ingested it would often start vomiting. Some physicians experimented with injections of the oil, but this produced painful pustules.

The Ball Method
If researchers could harness chaulmoogra’s curative potential without the nasty side effects, the tree’s seeds could revolutionize leprosy treatment. So, Hollmann turned to Ball. In a 1922 article, Hollmann documents how the 23-year-old Ball discovered how to chemically adapt chaulmoogra into an injection that had none of the side effects.
The Ball Method, as Hollmann called her discovery, transformed chaulmoogra oil into the most effective treatment for leprosy until the introduction of sulfones in the late 1940s.
In 1920, the Ball Method successfully treated 78 patients in Honolulu. A year later, it treated 94 more, with the Public Health Service noting that the morale of all the patients drastically improved. For the first time, there was hope for a cure.
Tragically, Ball did not have the opportunity to revel in this achievement, as she passed away within a year at only 24, likely from exposure to chlorine gas in the lab.
Ball’s legacy, lost and found
Ball’s death meant she didn’t have the opportunity to publish her research. Arthur Dean, chair of the College of Hawaii’s chemistry department, took over the project.
Dean mass-produced the treatment and published a series of articles on chaulmoogra oil. He renamed Ball’s method the “Dean Method,” and he never credited Ball for her work.
Ball’s other colleagues did attempt to protect Ball’s legacy. A 1920 article in the Journal of the American Medical Association praises the Ball Method, while Hollmann clearly credits Ball in his own 1922 article.
Ball is described at length in a 1922 article in volume 15, issue 5, of Current History, an academic publication on international affairs. That feature is excerpted in a June 1941 issue of Carter G. Woodson’s “Negro History Bulletin,” referring to Ball’s achievement and untimely death.
Joseph Dutton, a well-regarded religious volunteer at the leprosy settlements on Molokai, further referenced Ball’s work in a 1932 memoir broadly published for a popular audience.
Historians such as Paul Wermager later prompted a modern reckoning with Ball’s poor treatment by Dean and others, ensuring that Ball received proper credit for her work. Following Wermager’s and others’ work, the University of Hawaii honored Ball in 2000 with a bronze plaque, affixed to the last remaining chaulmoogra tree on campus.
In 2019, the London School of Hygiene and Tropical Medicine added Ball’s name to the outside of its building. Ball’s story was even featured in a 2020 short film, “The Ball Method.”
The Ball Method represents both a scientific achievement and a history of marginalization. A young woman of color pioneered a medical treatment for a highly stigmatizing disease that disproportionately affected an already disenfranchised Indigenous population.
In 2022, then-Gov. David Ige declared Feb. 28 Alice Augusta Ball Day in Hawaii. It was only fitting that the ceremony took place on the Mānoa campus in the shade of the chaulmoogra tree.
Mark M. Lambert, Assistant Professor of Behavioral Medicine, Medical Humanities, and Bioethics, Des Moines University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Read More