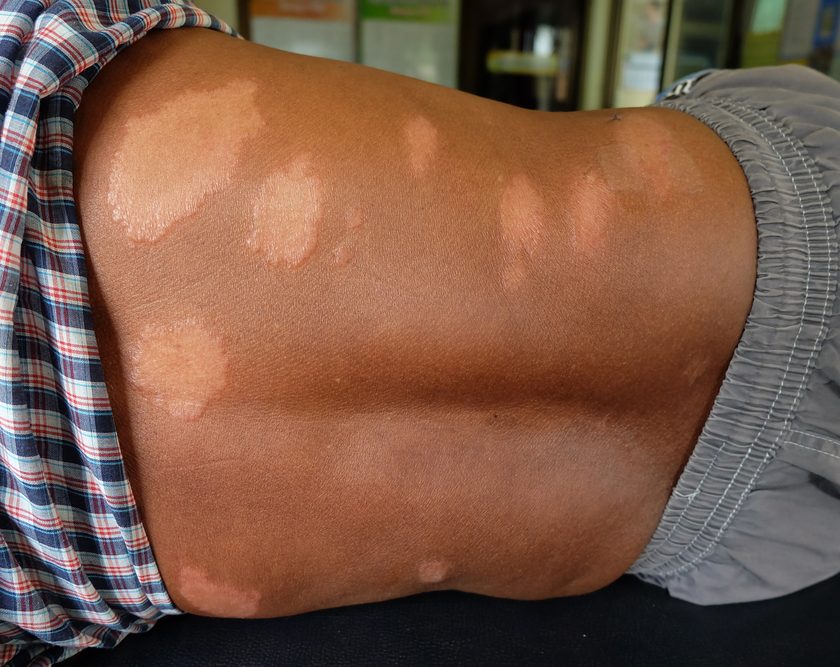
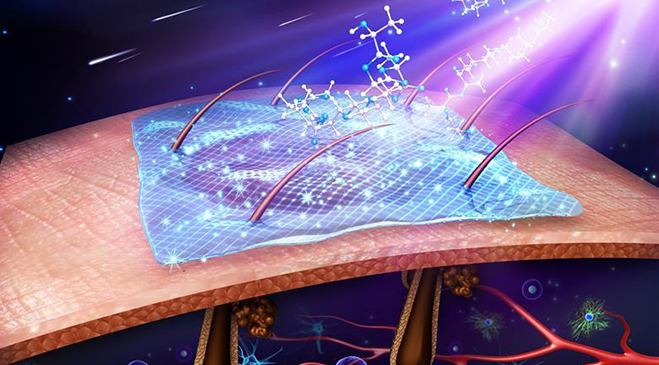
ROLLA, Mo.–(BUSINESS WIRE)–ETS Wound Care LLC, an Engineered Tissue Solutions (ETS) subsidiary focused on commercializing next generation wound care solutions, announced MIRRAGEN™ Advanced Wound Matrix was cleared by the United States Food and Drug Administration (FDA) for treatment of acute and chronic wounds. MIRRAGEN™ is a fully resorbable borate glass matrix comprised of fibers and beads proven to be highly effective in wound care management.
MIRRAGEN™ represents a breakthrough discovery for chronic and acute wound management due to its unique borate-based fiber matrix. MIRRAGEN™ is packed into wounds to manage and control wound fluids, while the resorbable matrix provides an environment for optimal wound healing. To learn more about the technology, click here.
“Anyone who is treating or experiencing discomfort from acute or chronic wounds will immediately recognize the benefits of MIRRAGEN™,” said Peggy Earl, BSN, RN, WOCN, a wound care specialist at Phelps County Regional Medical Center located in Rolla, Missouri. “MIRRAGEN™ has the potential to reduce the required number of episodes and duration of wound care treatment, while allowing the body to effectively heal a variety of wounds, both acute and chronic.”
“The recent FDA approval is a significant milestone for ETS,” said Chad Lewis, PhD, ETS Wound Care President & CEO. “ETS Wound Care is pioneering an entirely new therapeutic option for wound care. The MIRRAGEN™ Advanced Wound Matrix is the first product utilizing our innovative borate fiber technology engineered to improve patient outcomes.”
ETS Wound Care plans to make MIRRAGEN™ commercially available via a controlled domestic market release in early Q2 2017 and available to the broader domestic market in 2018. Later this week, the Company will be an exhibitor at the Nation’s largest interdisciplinary wound care forum – the Symposium on Advanced Wound Care Spring meeting (SAWC Spring) from April 6-8, 2017 in San Diego. Additional product information will be available at the ETS Wound Care exhibit booth (#602).
About ETS Wound Care LLC
ETS Wound Care, a subsidiary company of Engineered Tissue Solutions, is an emerging wound care company focused on using ETS’s borate-based bioactive technology to develop and commercialize a broad range of breakthrough solutions for the wound care market to minimize the cost of treatment and improve patient outcomes. Learn more at etswoundcare.com.
About Engineered Tissue Solutions (ETS)
ETS is a holdings company focused on “Healing People to Help People” by leveraging the borate-based bioactive glass technologies conceived and developed by researchers at Missouri University of Science and Technology (Missouri S&T) and Mo-Sci Corporation, both of Rolla, Missouri. In addition to providing breakthrough technologies for healthcare applications, ETS is committed to donating a portion of its profits to charity. For more information about ETS, visit etissuesolutions.com.
Contacts
ETS Wound Care LLC
Chad Lewis
President & CEO
[email protected]